Abstract
Purpose of Review
Stroke remains a leading disabling condition, and many survivors have permanent disability despite acute stroke treatment and subsequent standard-of-care rehabilitation therapies. Adjunctive neuromodulation is an emerging frontier in the field of stroke recovery. In this narrative review, we aim to highlight and summarize various neuromodulation techniques currently being investigated to enhance recovery and reduce impairment in patients with stroke.
Recent Findings
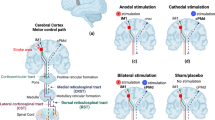
For motor recovery, repetitive transcranial magnetic simulation (rTMS) and direct current stimulation (tDCS) have shown promising results in many smaller-scale trials. Still, their efficacy has yet to be proven in large-scale pivotal trials. A promising large-scale study investigating higher dose tDCS combined with constraint movement therapy to enhance motor recovery is currently underway. MRI-guided tDCS studies in subacute and chronic post-stroke aphasia showed promising benefits for picture-naming recovery. rTMS, particularly inhibitory stimulation over the contralesional homolog, could represent a pathway forward in post-stroke motor recovery in the setting of a well-designed and adequately powered clinical trial. Recently evidenced-based guideline actually supported Level A (definite efficacy) for the use of low-frequency rTMS of the primary motor cortex for hand motor recovery in the post-acute stage of stroke based on the meta-analysis result. Adjunctive vagal nerve stimulation has recently received FDA approval to enhance upper limb motor recovery in chronic ischemic stroke with moderate impairment, and progress has been made to implement it in real-world practice. Despite a few small and large-scale studies in epidural stimulation (EDS), further research on the utilization of EDS in post-stroke recovery is needed. Deep brain stimulation or stent-based neuromodulation has yet to be further tested regarding safety and efficacy.
Summary
Adjunctive neuromodulation to rehabilitation therapy is a promising avenue for promoting post-stroke recovery and decreasing the overall burden of disability. The pipeline for neuromodulation technology remains strong as they span from the preclinical stage to the post-market stage. We are optimistic to see that more neuromodulation tools will be available to stroke survivors in the not-to-distant future.

Similar content being viewed by others
Data Availability
The principal investigator, Dr. Wuwei Feng, takes full responsibility for the data, analyses and interpretations, and the conduct of the research. Dr. Feng has full access to all of the data and has the right to publish any and all data separate and apart from any sponsor.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Tsao CW, et al. Heart disease and stroke statistics—2023 update: a report from the American Heart Association. Circulation. 2023;147(8):e93–621.
Boyd LA, et al. Biomarkers of stroke recovery: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int J Stroke. 2017;12(5):480–93.
Feng W, et al. Corticospinal tract lesion load: an imaging biomarker for stroke motor outcomes. Ann Neurol. 2015;78(6):860–70. This manuscript developed an imaging biomarker using a routine clinical MRI scan collected in the first 7 days, and it can effectively predict upper extremity impairment at 90 days. Lesion volume of 7.0cc is a critical threshold to have poor recovery in upper extremity.
Krakauer JW, Marshall RS. The proportional recovery rule for stroke revisited. Ann Neurol. 2015;78(6):845–7.
Keser Z, et al. Electroencephalogram (EEG) with or without transcranial magnetic stimulation (TMS) as biomarkers for post-stroke recovery: a narrative review. Front Neurol. 2022;13:827866.
Nowak DA, et al. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair. 2009;23(7):641–56.
Di Pino G, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol. 2014;10(10):597–608.
Dromerick, A.W., et al. Critical Period After Stroke Study (CPASS): a phase II clinical trial testing an optimal time for motor recovery after stroke in humans. Proc Natl Acad Sci U S A 2021; 118(39)
Emara TH, et al. Repetitive transcranial magnetic stimulation at 1Hz and 5Hz produces sustained improvement in motor function and disability after ischaemic stroke. Eur J Neurol. 2010;17(9):1203–9.
Takeuchi N, et al. Inhibition of the unaffected motor cortex by 1 Hz repetitive transcranical magnetic stimulation enhances motor performance and training effect of the paretic hand in patients with chronic stroke. J Rehabil Med. 2008;40(4):298–303.
Harvey RL, et al. Randomized sham-controlled trial of navigated repetitive transcranial magnetic stimulation for motor recovery in stroke. Stroke. 2018;49(9):2138–46. The review paper proposed a bimodal balance-recovery model that links interhemispheric balancing and functional recovery to the structural reserve spared by the lesion.
Kim WS, et al. Low-frequency repetitive transcranial magnetic stimulation over contralesional motor cortex for motor recovery in subacute ischemic stroke: a randomized sham-controlled trial. Neurorehabil Neural Repair. 2020;34(9):856–67.
Lefaucheur JP, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin Neurophysiol. 2020;131(2):474–528.
Vink JJT, et al. Continuous theta-burst stimulation of the contralesional primary motor cortex for promotion of upper limb recovery after stroke: a randomized controlled trial. Stroke. 2023;54(8):1962–71. This is single-center study demonstrated continuous theta burst stimulation of unaffected hemispheric can reduce motor impairment, improve function and enhance quality of life in a cohort of subacute stroke patients with upper extremity impairment.
Feng W, Plow EB, Paik NJ. Transcranial magnetic stimulation for poststroke motor recovery: what we have learned. Stroke. 2023;54(8):1972–3.
Chiu D, et al. Multifocal transcranial stimulation in chronic ischemic stroke: a phase 1/2a randomized trial. J Stroke Cerebrovasc Dis. 2020;29(6):104816.
Gholami M, Pourbaghi N, Taghvatalab S. Evaluation of rTMS in patients with poststroke aphasia: a systematic review and focused meta-analysis. Neurol Sci. 2022;43(8):4685–94.
Fu W, et al. Long-term effects of continuous theta-burst stimulation in visuospatial neglect. J Int Med Res. 2015;43(2):196–203.
Nyffeler T, et al. Theta burst stimulation in neglect after stroke: functional outcome and response variability origins. Brain. 2019;142(4):992–1008.
Cheng IKY, et al. Neuronavigated high-frequency repetitive transcranial magnetic stimulation for chronic post-stroke dysphagia: a randomized controlled study. J Rehabil Med. 2017;49(6):475–81.
Zhang C, et al. Repetitive transcranial magnetic stimulation in combination with neuromuscular electrical stimulation for treatment of post-stroke dysphagia. J Int Med Res. 2019;47(2):662–72.
Rossi C, et al. Transcranial direct current stimulation of the affected hemisphere does not accelerate recovery of acute stroke patients. Eur J Neurol. 2013;20(1):202–4.
Sattler V, et al. Anodal tDCS combined with radial nerve stimulation promotes hand motor recovery in the acute phase after ischemic stroke. Neurorehabil Neural Repair. 2015;29(8):743–54.
Hesse S, et al. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: an exploratory, randomized multicenter trial. Neurorehabil Neural Repair. 2011;25(9):838–46.
Khedr EM, et al. Effect of anodal versus cathodal transcranial direct current stimulation on stroke rehabilitation: a pilot randomized controlled trial. Neurorehabil Neural Repair. 2013;27(7):592–601.
Chang MC, Kim DY, Park DH. Enhancement of cortical excitability and lower limb motor function in patients with stroke by transcranial direct current stimulation. Brain Stimul. 2015;8(3):561–6.
Geroin C, et al. Combined transcranial direct current stimulation and robot-assisted gait training in patients with chronic stroke: a preliminary comparison. Clin Rehabil. 2011;25(6):537–48.
Viana RT, et al. Effects of the addition of transcranial direct current stimulation to virtual reality therapy after stroke: a pilot randomized controlled trial. NeuroRehabilitation. 2014;34(3):437–46.
Allman C, et al. Ipsilesional anodal tDCS enhances the functional benefits of rehabilitation in patients after stroke. Sci Transl Med. 2016;8(330):330re1.
Vöröslakos M, et al. Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nat Commun. 2018;9(1):483.
Marangolo P, et al. tDCS over the left inferior frontal cortex improves speech production in aphasia. Front Hum Neurosci. 2013;7:539.
Campana S, Caltagirone C, Marangolo P. Combining voxel-based lesion-symptom mapping (VLSM) with A-tDCS language treatment: predicting outcome of recovery in nonfluent chronic aphasia. Brain Stimul. 2015;8(4):769–76.
Polanowska KE, et al. Anodal transcranial direct current stimulation in early rehabilitation of patients with post-stroke non-fluent aphasia: a randomized, double-blind, sham-controlled pilot study. Restor Neurol Neurosci. 2013;31(6):761–71.
Keser Z, et al. Combined dextroamphetamine and transcranial direct current stimulation in poststroke aphasia. Am J Phys Med Rehabil. 2017;96(10):S141–5.
Keser Z, Francisco GE. Neuromodulation for post-stroke aphasia. Curr Phys Med Rehabil Rep. 2016;4(3):171–81.
Fridriksson J, et al. Transcranial direct current stimulation vs sham stimulation to treat aphasia after stroke: a randomized clinical trial. JAMA Neurol. 2018;75(12):1470–6.
Stockbridge MD, et al. Transcranial direct-current stimulation in subacute aphasia: a randomized controlled trial. Stroke. 2023;54(4):912–20.
Ko MH, et al. Home-based transcranial direct current stimulation to enhance cognition in stroke: randomized controlled trial. Stroke. 2022;53(10):2992–3001.
Bucak, B, Z. Keser. A commentary on: High-definition cathodal direct current stimulation for treatment of acute ischemic stroke: a randomized clinical trial. 2023 [cited 2023 07/15/2023]; Available from: https://www.practiceupdate.com/content/high-definition-cathodal-direct-current-stimulation-for-the-treatment-of-acute-ischemic-stroke/153641/65/7/1.
Pruvost-Robieux E, et al. Cathodal transcranial direct current stimulation in acute ischemic stroke: pilot randomized controlled trial. Stroke. 2021;52(6):1951–60.
Bahr-Hosseini M, et al. High-definition cathodal direct current stimulation for treatment of acute ischemic stroke: a randomized clinical trial. JAMA Netw Open. 2023;6(6):e2319231.
Feng W, et al. Transcranial direct current stimulation for poststroke motor recovery: challenges and opportunities. PM R. 2018;10(9 Suppl 2):S157–64. This review paper summarized all of the challenges, barriers and opportunities for tDCS to be a viable neuromodulatory tool for stroke recovery.
Li, K.P., et al. Noninvasive brain stimulation for neurorehabilitation in post-stroke patients. Brain Sci 2023; 13(3)
Chaieb L, Antal A, Paulus W. Transcranial alternating current stimulation in the low kHz range increases motor cortex excitability. Restor Neurol Neurosci. 2011;29(3):167–75.
Kanai R, Paulus W, Walsh V. Transcranial alternating current stimulation (tACS) modulates cortical excitability as assessed by TMS-induced phosphene thresholds. Clin Neurophysiol. 2010;121(9):1551–4.
Antal A, Paulus W. Transcranial alternating current stimulation (tACS). Front Hum Neurosci. 2013;7:317.
Wu JF, et al. Efficacy of transcranial alternating current stimulation over bilateral mastoids (tACS(bm)) on enhancing recovery of subacute post-stroke patients. Top Stroke Rehabil. 2016;23(6):420–9.
Fedorov A, et al. Non-invasive alternating current stimulation induces recovery from stroke. Restor Neurol Neurosci. 2010;28(6):825–33.
Baczyk M, et al. Long-lasting modifications of motoneuron firing properties by trans-spinal direct current stimulation in rats. Eur J Neurosci. 2020;51(8):1743–55.
Sasada S, et al. Polarity-dependent improvement of maximal-effort sprint cycling performance by direct current stimulation of the central nervous system. Neurosci Lett. 2017;657:97–101.
Marangolo, P., et al. What else can be done by the spinal cord? A review on the effectiveness of transpinal direct current stimulation (tsDCS) in stroke recovery. Int J Mol Sci 2023; 24(12)
Picelli A, et al. Combined effects of transcranial direct current stimulation (tDCS) and transcutaneous spinal direct current stimulation (tsDCS) on robot-assisted gait training in patients with chronic stroke: a pilot, double blind, randomized controlled trial. Restor Neurol Neurosci. 2015;33(3):357–68.
Paget-Blanc A, et al. Non-invasive treatment of patients with upper extremity spasticity following stroke using paired trans-spinal and peripheral direct current stimulation. Bioelectron Med. 2019;5:11.
Marangolo P, et al. Moving beyond the brain: transcutaneous spinal direct current stimulation in post-stroke aphasia. Front Neurol. 2017;8:400.
Marangolo P, et al. Stairways to the brain: transcutaneous spinal direct current stimulation (tsDCS) modulates a cerebellar-cortical network enhancing verb recovery. Brain Res. 2020;1727:146564.
Baek H, et al. Modulation of cerebellar cortical plasticity using low-intensity focused ultrasound for poststroke sensorimotor function recovery. Neurorehabil Neural Repair. 2018;32(9):777–87.
Ichijo S, et al. Low-intensity pulsed ultrasound therapy promotes recovery from stroke by enhancing angio-neurogenesis in mice in vivo. Sci Rep. 2021;11(1):4958.
Segal Y, et al. The effect of electromagnetic field treatment on recovery from ischemic stroke in a rat stroke model: clinical, imaging, and pathological findings. Stroke Res Treat. 2016;2016:6941946.
Weisinger B, et al. Frequency-tuned electromagnetic field therapy improves post-stroke motor function: a pilot randomized controlled trial. Front Neurol. 2022;13:1004677.
Vosler PS, et al. Mitochondrial targets for stroke: focusing basic science research toward development of clinically translatable therapeutics. Stroke. 2009;40(9):3149–55.
Feng W, et al. Revisiting transcranial light stimulation as a stroke therapeutic-hurdles and opportunities. Transl Stroke Res. 2022. https://doi.org/10.1007/s12975-022-01103-7.
Dawson J, et al. Safety, feasibility, and efficacy of vagus nerve stimulation paired with upper-limb rehabilitation after ischemic stroke. Stroke. 2016;47(1):143–50.
Kimberley TJ, et al. Vagus nerve stimulation paired with upper limb rehabilitation after chronic stroke. Stroke. 2018;49(11):2789–92.
Dawson J, et al. Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): a randomised, blinded, pivotal, device trial. Lancet. 2021;397(10284):1545–53. In this first in human pilot study, adjunctive deep brain stimulation led to notable improvements in upper limb functions in chronic stroke.
Keser Z, Feng W. Vagus nerve stimulation for stroke motor recovery—what is next? Transl Stroke Res. 2023;14(4):438–42.
Bahr-Hosseini M, Saver JL. Mechanisms of action of acute and subacute sphenopalatine ganglion stimulation for ischemic stroke. Int J Stroke. 2020;15(8):839–48.
Bornstein NM, et al. Sphenopalatine ganglion stimulation to augment cerebral blood flow: a randomized, sham-controlled trial. Stroke. 2019;50(8):2108–17.
Bornstein NM, et al. An injectable implant to stimulate the sphenopalatine ganglion for treatment of acute ischaemic stroke up to 24 h from onset (ImpACT-24B): an international, randomised, double-blind, sham-controlled, pivotal trial. Lancet. 2019;394(10194):219–29.
Wathen CA, et al. Deep brain stimulation of the cerebellum for poststroke motor rehabilitation: from laboratory to clinical trial. Neurosurg Focus. 2018;45(2):E13.
Keser Z, et al. Diffusion tensor imaging of the human cerebellar pathways and their interplay with cerebral macrostructure. Front Neuroanat. 2015;9:41.
Baker KB, et al. Cerebellar deep brain stimulation for chronic post-stroke motor rehabilitation: a phase I trial. Nat Med. 2023;29.9:2366-74. https://doi.org/10.1038/s41591-023-02507-0
Brown JA, et al. Motor cortex stimulation for the enhancement of recovery from stroke: a prospective, multicenter safety study. Neurosurgery. 2006;58(3):464–73.
Cherney LR, Erickson RK, Small SL. Epidural cortical stimulation as adjunctive treatment for non-fluent aphasia: preliminary findings. J Neurol Neurosurg Psychiatry. 2010;81(9):1014–21.
Cherney LR. Epidural cortical stimulation as adjunctive treatment for nonfluent aphasia: phase 1 clinical trial follow-up findings. Neurorehabil Neural Repair. 2016;30(2):131–42.
Levy RM, et al. Epidural electrical stimulation for stroke rehabilitation: results of the prospective, multicenter, randomized, single-blinded everest trial. Neurorehabil Neural Repair. 2016;30(2):107–19.
Powell MP, et al. Epidural stimulation of the cervical spinal cord for post-stroke upper-limb paresis. Nat Med. 2023;29(3):689–99. This pilot study revealed EDS of the cervical spinal cord was feasible, appeared to induce an increase in grip strength and movement kinematics of upper limb.
Opie NL, et al. Focal stimulation of the sheep motor cortex with a chronically implanted minimally invasive electrode array mounted on an endovascular stent. Nat Biomed Eng. 2018;2(12):907–14.
Mitchell P, et al. Assessment of safety of a fully implanted endovascular brain-computer interface for severe paralysis in 4 patients: the Stentrode With Thought-Controlled Digital Switch (SWITCH) study. JAMA Neurol. 2023;80(3):270–8. This proof-of-concept study showed safety and preliminary efficacy of endovascular brain-computer interface (BCI) system placed in superior sagittal sinus in five patients with advanced motor neuron disease and severe bilateral arm weakness.
Alawieh A, Fernando Gonzalez L, Feng W. Barriers and opportunities of cortical stimulation via cerebral venous approach. Brain Stimul. 2020;13(2):401–2.
Author information
Authors and Affiliations
Contributions
ZK conducted the review and wrote the manuscript text. SI prepared the figures, tables, and edited the main text of the manuscript. SS reviewed and edited the main text of the manuscript. WF designed the study and edited the main text of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This review article does not contain any studies with human or animal subjects performed by any author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Keser, Z., Ikramuddin, S., Shekhar, S. et al. Neuromodulation for Post-Stroke Motor Recovery: a Narrative Review of Invasive and Non‑Invasive Tools. Curr Neurol Neurosci Rep 23, 893–906 (2023). https://doi.org/10.1007/s11910-023-01319-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-023-01319-6