Abstract
Purpose of Review
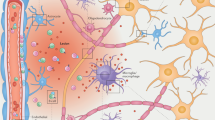
Multiple sclerosis is characterized by a diverse and complex pathology. Clinical relapses, the hallmark of the disease, are accompanied by focal white matter lesions with intense inflammatory and demyelinating activity. Prevention of these relapses has been the major focus of pharmaceutical development, and it is now possible to dramatically reduce this inflammatory activity. Unfortunately, disability accumulation persists for many people living with multiple sclerosis owing to ongoing damage within existing lesions, pathology outside of discrete lesions, and other yet unknown factors. Understanding this complex pathological cascade will be critical to stopping progressive multiple sclerosis. Positron emission tomography uses biochemically specific radioligands to quantitatively measure pathological processes with molecular specificity. This review examines recent advances in the understanding of multiple sclerosis facilitated by positron emission tomography and identifies future avenues to expand understanding and treatment options.
Recent Findings
An increasing number of radiotracers allow for the quantitative measurement of inflammatory abnormalities, de- and re-myelination, and metabolic disruption associated with multiple sclerosis. The studies have identified contributions of ongoing, smoldering inflammation to accumulating tissue injury and clinical worsening. Myelin studies have quantified the dynamics of myelin loss and recovery. Lastly, metabolic changes have been found to contribute to symptom worsening.
Summary
The molecular specificity facilitated by positron emission tomography in people living with multiple sclerosis will critically inform efforts to modulate the pathology leading to progressive disability accumulation. Existing studies show the power of this approach applied to multiple sclerosis. This armamentarium of radioligands allows for new understanding of how the brain and spinal cord of people is impacted by multiple sclerosis.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hemond CC, Bakshi R. Magnetic resonance imaging in multiple sclerosis. Cold Spring Harb Perspect Med. 2018;8:a028969.
Bodini B, Tonietto M, Airas L, Stankoff B. Positron emission tomography in multiple sclerosis — straight to the target. Nat Rev Neurol. 2021;17:663–75.
Absinta M, Vuolo L, Rao A, Nair G, Sati P, Cortese ICM, et al. Gadolinium-based MRI characterization of leptomeningeal inflammation in multiple sclerosis. Neurology. 2015;85:18–28.
Maggi P, Sati P, Nair G, Cortese ICM, Jacobson S, Smith BR, et al. Paramagnetic rim lesions are specific to multiple sclerosis: an international multicenter 3T MRI study. Ann Neurol. 2020;88:1034–42.
Veronese M, Bodini B, García-Lorenzo D, Battaglini M, Bongarzone S, Comtat C, et al. Quantification of [11C]PIB PET for imaging myelin in the human brain: a test—retest reproducibility study in high-resolution research tomography. J Cereb Blood Flow Metab. 2015;35:1771–82.
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol (Berl). 2017;133:13–24.
Lassmann H. The pathology of multiple sclerosis and its evolution. McDonald WI, editor. Philos Trans R Soc Lond B Biol ScI. 1999;3:1635–40.
Matthews PM. Chronic inflammation in multiple sclerosis — seeing what was always there. Nat Rev Neurol. 2019;15:582–93.
Elliott C, Wolinsky JS, Hauser SL, Kappos L, Barkhof F, Bernasconi C, et al. Slowly expanding/evolving lesions as a magnetic resonance imaging marker of chronic active multiple sclerosis lesions. Mult Scler J. 2019;25:1915–25.
Calvi A, Haider L, Prados F, Tur C, Chard D, Barkhof F. In vivo imaging of chronic active lesions in multiple sclerosis. Mult Scler J. 2022;28:683–90.
Absinta M, Sati P, Masuzzo F, Nair G, Sethi V, Kolb H, et al. Association of chronic active multiple sclerosis lesions with disability in vivo. JAMA Neurol. 2019;76:1474–83.
Seewann A, Kooi E-J, Roosendaal SD, Barkhof F, Van Der Valk P, Geurts JJG. Translating pathology in multiple sclerosis: the combination of postmortem imaging, histopathology and clinical findings. Acta Neurol Scand. 2009;119:349–55.
Bagnato F, Jeffries N, Richert ND, Stone RD, Ohayon JM, McFarland HF, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782–9.
Sahraian MA, Radue E-W, Haller S, Kappos L. Black holes in multiple sclerosis: definition, evolution, and clinical correlations. Acta Neurol Scand. 2010;122:1–8.
Truyen L, van Waesberghe JHTM, van Walderveen MAA, van Oosten BW, Polman CH, Hommes OR, et al. Accumulation of hypointense lesions (“black holes”) on T1 spin-echo MRI correlates with disease progression in multiple sclerosis. Neurology. 1996;47:1469–76.
Van Der Weijden CWJ, Biondetti E, Gutmann IW, Dijkstra H, McKerchar R, De Paula FD, et al. Quantitative myelin imaging with MRI and PET: an overview of techniques and their validation status. Brain. 2023;146:1243–66.
Moll NM, Rietsch AM, Thomas S, Ransohoff AJ, Lee J-C, Fox R, et al. Multiple sclerosis normal-appearing white matter: pathology–imaging correlations. Ann Neurol. 2011;70:764–73.
Brier MR, Snyder AZ, Tanenbaum A, Rudick RA, Fisher E, Jones S, et al. Quantitative signal properties from standardized MRIs correlate with multiple sclerosis disability. Ann Clin Transl Neurol. 2021;8:1096–109.
Laule C, Pavlova V, Leung E, Zhao G, MacKay AL, Kozlowski P, et al. Diffusely abnormal white matter in multiple sclerosis: further histologic studies provide evidence for a primary lipid abnormality with neurodegeneration. J Neuropathol Exp Neurol. 2013;72:42–52.
Ge Y, Grossman RI, Babb JS, He J, Mannon LJ. Dirty-appearing white matter in multiple sclerosis: volumetric MR imaging and magnetization transfer ratio histogram analysis. AJNR Am J Neuroradiol. 2003;24:1935–40.
Laule C, Vavasour IM, Leung E, Li DK, Kozlowski P, Traboulsee AL, et al. Pathological basis of diffusely abnormal white matter: insights from magnetic resonance imaging and histology. Mult Scler J. 2011;17:144–50.
Xiang B, Wen J, Schmidt RE, Sukstanskii AL, Mamah D, Yablonskiy DA, et al. Evaluating brain damage in multiple sclerosis with simultaneous multi-angular-relaxometry of tissue. Ann Clin Transl Neurol. 2022;9:1514–27.
Bö L, Geurts JJG, Mörk SJ, van der Valk P. Grey matter pathology in multiple sclerosis. Acta Neurol Scand. 2006;113:48–50.
Vercellino M, Plano F, Votta B, Mutani R, Giordana MT, Cavalla P. Grey Matter pathology in multiple sclerosis. J Neuropathol Exp Neurol. 2005;64:1101–7.
Calabrese M, Filippi M, Gallo P. Cortical lesions in multiple sclerosis. Nat Rev Neurol. 2010;6:438–44.
Calabrese M, Battaglini M, Giorgio A, Atzori M, Bernardi V, Mattisi I, et al. Imaging distribution and frequency of cortical lesions in patients with multiple sclerosis. Neurology. 2010;75:1234–40.
Calabrese M, Poretto V, Favaretto A, Alessio S, Bernardi V, Romualdi C, et al. Cortical lesion load associates with progression of disability in multiple sclerosis. Brain. 2012;135:2952–61.
Kilsdonk ID, Jonkman LE, Klaver R, van Veluw SJ, Zwanenburg JJM, Kuijer JPA, et al. Increased cortical grey matter lesion detection in multiple sclerosis with 7 T MRI: a post-mortem verification study. Brain. 2016;139:1472–81.
Wicken C, Nguyen J, Karna R, Bhargava P. Leptomeningeal inflammation in multiple sclerosis: Insights from animal and human studies. Mult Scler Relat Disord. 2018;26:173–82.
Harrison DM, Wang KY, Fiol J, Naunton K, Royal W III, Hua J, et al. Leptomeningeal enhancement at 7T in multiple sclerosis: frequency, morphology, and relationship to cortical volume. J Neuroimaging. 2017;27:461–8.
Zurawski J, Lassmann H, Bakshi R. Use of magnetic resonance imaging to visualize leptomeningeal inflammation in patients with multiple sclerosis: a review. JAMA Neurol. 2017;74:100–9.
Chételat G, Arbizu J, Barthel H, Garibotto V, Law I, Morbelli S, et al. Amyloid-PET and 18F-FDG-PET in the diagnostic investigation of Alzheimer’s disease and other dementias. Lancet Neurol. 2020;19:951–62.
Van Der Aart J, Hallett WA, Rabiner EA, Passchier J, Comley RA. Radiation dose estimates for carbon-11-labelled PET tracers. Nucl Med Biol. 2012;39:305–14.
Rossano S, Toyonaga T, Bini J, Nabulsi N, Ropchan J, Cai Z, et al. Feasibility of imaging synaptic density in the human spinal cord using [11C]UCB-J PET. EJNMMI Phys. 2022;9:32. This study uses a synaptic vesicle-associated tracer to measure synaptic density in the spinal cord. While not an MS study, this study demonstrates that PET in the spinal cord is possible and opens entirely new avenues of investigation to the MS research community.
Thomas BA, Cuplov V, Bousse A, Mendes A, Thielemans K, Hutton BF, et al. PETPVC: a toolbox for performing partial volume correction techniques in positron emission tomography. Phys Med Biol. 2016;61:7975.
Reivich M, Alavi A, Wolf A, Fowler J, Russell J, Arnett C, et al. Glucose metabolic rate kinetic model parameter determination in humans: the lumped constants and rate constants for [18F]fluorodeoxyglucose and [11C]deoxyglucose. J Cereb Blood Flow Metab. 1985;5:179–92.
Maffione AM, Rampin L, Grassetto G, L’Erario R, Colletti PM, Rubello D. 18F-FDG PET/CT in tumefactive multiple sclerosis. Clin Nucl Med. 2014;39:750–1.
Schiepers C, Van Hecke P, Vandenberghe R, Van Oostende S, Dupont P, Demaerel P, et al. Positron emission tomography, magnetic resonance imaging and proton NMR spectroscopy of white matter in multiple sclerosis. Mult Scler J. 1997;3:8–17.
Derache N, Marié R-M, Constans J-M, Defer G-L. Reduced thalamic and cerebellar rest metabolism in relapsing–remitting multiple sclerosis, a positron emission tomography study: correlations to lesion load. J Neurol Sci. 2006;245:103–9.
Blinkenberg M, Jensen CV, Holm S, Paulson OB, Sorensen PS. A longitudinal study of cerebral glucose metabolism, MRI, and disability in patients with MS. Neurology. 1999;53:149–149.
Baumgartner A, Frings L, Schiller F, Stich O, Mix M, Egger K, et al. Regional neuronal activity in patients with relapsing remitting multiple sclerosis. Acta Neurol Scand. 2018;138:466–74.
Paulesu E, Perani D, Fazio F, Comi G, Pozzilli C, Martinelli V, et al. Functional basis of memory impairment in multiple sclerosis: a [18F]FDG PET study. Neuroimage. 1996;4:87–96.
Blinkenberg M, Rune K, Jensen CV, Ravnborg M, Kyllingsbak S, Holm S, et al. Cortical cerebral metabolism correlates with MRI lesion load and cognitive dysfunction in MS. Neurology. 2000;54:558–558.
Roelcke U, Kappos L, Lechner-Scott J, Brunnschweiler H, Huber S, Ammann W, et al. Reduced glucose metabolism in the frontal cortex and basal ganglia of multiple sclerosis patients with fatigue: a 18 F-fluorodeoxyglucose positron emission tomography study. Neurology. 1997;48:1566–71.
Brooks DJ, Leenders KL, Head G, Marshall J, Legg NJ, Jones T. Studies on regional cerebral oxygen utilisation and cognitive function in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1984;47:1182–91.
Sun X, Tanaka M, Kondo S, Okamoto K, Hirai S. Clinical significance of reduced cerebral metabolism in multiple sclerosis: a combined PET and MRI study. Ann Nucl Med. 1998;12:89–94.
Trapp BD, Stys PK. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 2009;8:280–91.
Nave K-A. Myelination and the trophic support of long axons. Nat Rev Neurosci. 2010;11:275–83.
Vowinckel E, Reutens D, Becher B, Verge G, Evans A, Owens T, et al. PK11195 binding to the peripheral benzodiazepine receptor as a marker of microglia activation in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neurosci Res. 1997;50:345–53.
Rissanen E, Tuisku J, Vahlberg T, Sucksdorff M, Paavilainen T, Parkkola R, et al. Microglial activation, white matter tract damage, and disability in MS. Neurol - Neuroimmunol Neuroinflammation [Internet]. 2018 [cited 2023 Apr 10];5. Available from: https://doi.org/10.1212/NXI.0000000000000443
Sucksdorff M, Matilainen M, Tuisku J, Polvinen E, Vuorimaa A, Rokka J, et al. Brain TSPO-PET predicts later disease progression independent of relapses in multiple sclerosis. Brain. 2020;143:3318–30.
Rissanen E, Tuisku J, Rokka J, Paavilainen T, Parkkola R, Rinne JO, et al. In vivo detection of diffuse inflammation in secondary progressive multiple sclerosis using PET imaging and the radioligand 11C-PK11195. J Nucl Med. 2014;55:939–44.
Kang Y, Pandya S, Zinger N, Michaelson N, Gauthier SA. Longitudinal change in TSPO PET imaging in progressive multiple sclerosis. Ann Clin Transl Neurol. 2021;8:1755–9. Microglia number measured by TSPO PET increases over time in patients with progressive MS.
Debruyne JC, Versijpt J, Van Laere KJ, De Vos F, Keppens J, Strijckmans K, et al. PET visualization of microglia in multiple sclerosis patients using [11C]PK11195. Eur J Neurol. 2003;10:257–64.
Versijpt J, Debruyne JC, Van Laere KJ, De Vos F, Keppens J, Strijckmans K, et al. Microglial imaging with positron emission tomography and atrophy measurements with magnetic resonance imaging in multiple sclerosis: a correlative study. Mult Scler J. 2005;11:127–34.
Giannetti P, Politis M, Su P, Turkheimer F, Malik O, Keihaninejad S, et al. Microglia activation in multiple sclerosis black holes predicts outcome in progressive patients: an in vivo [(11)C](R)-PK11195-PET pilot study. Neurobiol Dis. 2014;65:203–10.
Giannetti P, Politis M, Su P, Turkheimer FE, Malik O, Keihaninejad S, et al. Increased PK11195-PET binding in normal-appearing white matter in clinically isolated syndrome. Brain. 2015;138:110–9.
Banati RB, Newcombe J, Gunn RN, Cagnin A, Turkheimer F, Heppner F, et al. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain. 2000;123:2321–37.
Misin O, Matilainen M, Nylund M, Honkonen E, Rissanen E, Sucksdorff M, et al. Innate immune cell-related pathology in the thalamus signals a risk for disability progression in multiple sclerosis. Neurol Neuroimmunol Neuroinflammation. 2022;9:e1182.
Sucksdorff M, Tuisku J, Matilainen M, Vuorimaa A, Smith S, Keitilä J, et al. Natalizumab treatment reduces microglial activation in the white matter of the MS brain. Neurol Neuroimmunol Neuroinflammation. 2019;6:e574.
Ratchford JN, Endres CJ, Hammoud DA, Pomper MG, Shiee N, McGready J, et al. Decreased microglial activation in MS patients treated with glatiramer acetate. J Neurol. 2012;259:1199–205.
Sucksdorff M, Rissanen E, Tuisku J, Nuutinen S, Paavilainen T, Rokka J, et al. Evaluation of the effect of fingolimod treatment on microglial activation using serial PET imaging in multiple sclerosis. J Nucl Med. 2017;58:1646–51.
Jučaite A, Cselényi Z, Arvidsson A, Åhlberg G, Julin P, Varnäs K, et al. Kinetic analysis and test-retest variability of the radioligand [11C](R)-PK11195 binding to TSPO in the human brain - a PET study in control subjects. EJNMMI Res. 2012;2:15.
Oh U, Fujita M, Ikonomidou VN, Evangelou IE, Matsuura E, Harberts E, et al. Translocator protein PET imaging for glial activation in multiple sclerosis. J Neuroimmune Pharmacol. 2011;6:354–61.
Park E, Gallezot J-D, Delgadillo A, Liu S, Planeta B, Lin S-F, et al. 11C-PBR28 imaging in multiple sclerosis patients and healthy controls: test-retest reproducibility and focal visualization of active white matter areas. Eur J Nucl Med Mol Imaging. 2015;42:1081–92.
Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab. 2012;32:1–5.
Herranz E, Giannì C, Louapre C, Treaba CA, Govindarajan ST, Ouellette R, et al. Neuroinflammatory component of gray matter pathology in multiple sclerosis. Ann Neurol. 2016;80:776–90.
Hamzaoui M, Garcia J, Boffa G, Lazzarotto A, Absinta M, Ricigliano VAG, et al. Positron emission tomography with [18F]-DPA-714 unveils a smoldering component in most multiple sclerosis lesions which drives disease progression. Ann Neurol [Internet]. [cited 2023 May 4];n/a. Available from: https://doi.org/10.1002/ana.26657. Demonstrates three phenotypes of microglia number in lesions: decreased, rim of increased microglia, and universally increased microglia. The presence of globally increase microglia number was associated with clinical progression.
Nutma E, Gebro E, Marzin MC, van der Valk P, Matthews PM, Owen DR, et al. Activated microglia do not increase 18 kDa translocator protein (TSPO) expression in the multiple sclerosis brain. Glia. 2021;69:2447–58.
Owen DRJ, Narayan N, Wells L, Healy L, Smyth E, Rabiner EA, et al. Pro-inflammatory activation of primary microglia and macrophages increases 18kDa translocator protein (TSPO) expression in rodents but not humans. 2690 [Internet]. 2017 [cited 2023 Apr 10]; Available from: https://doi.org/10.1177/0271678X17710182
Nutma E, Stephenson JA, Gorter RP, de Bruin J, Boucherie DM, Donat CK, et al. A quantitative neuropathological assessment of translocator protein expression in multiple sclerosis. Brain. 2019;142:3440–55.
Ponath G, Park C, Pitt D. The role of astrocytes in multiple sclerosis. Front Immunol [Internet]. 2018 [cited 2023 Apr 26];9. Available from: https://doi.org/10.3389/fimmu.2018.00217
Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung H-P, Hemmer B, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221–34.
Hauser SL, Bar-Or A, Cohen JA, Comi G, Correale J, Coyle PK, et al. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med. 2020;383:546–57.
James ML, Hoehne A, Mayer AT, Lechtenberg K, Moreno M, Gowrishankar G, et al. Imaging B cells in a mouse model of multiple sclerosis using 64Cu-rituximab PET. J Nucl Med. 2017;58:1845–51.
Guglielmetti C, Levi J, Huynh TL, Tiret B, Blecha J, Tang R, et al. Longitudinal imaging of T cells and inflammatory demyelination in a preclinical model of multiple sclerosis using 18F-FAraG PET and MRI. J Nucl Med. 2022;63:140–6.
Brier MR, Hamdi M, Rajamanikam J, Zhao H, Mansor S, Jones LA, et al. Phase 1 evaluation of 11C-CS1P1 to assess safety and dosimetry in human participants. J Nucl Med Off Publ Soc Nucl Med. 2022;63:1775–82.
Kato H, Okuno T, Isohashi K, Koda T, Shimizu M, Mochizuki H, et al. Astrocyte metabolism in multiple sclerosis investigated by 1-C-11 acetate PET. J Cereb Blood Flow Metab. 2021;41:369–79. Activated astrocytes are a known component of MS pathology and this paper demonstrates that acetate imaging is able to measure that activation. Increase astrocyte activation correlated with pathology measured with diffusion imaging.
Takata K, Kato H, Shimosegawa E, Okuno T, Koda T, Sugimoto T, et al. 11C-Acetate PET imaging in patients with multiple sclerosis. PLoS ONE. 2014;9:e111598.
Wu C, Tian D, Feng Y, Polak P, Wei J, Sharp A, et al. A novel fluorescent probe that is brain permeable and selectively binds to myelin. J Histochem Cytochem. 2006;54:997–1004.
Stankoff B, Wang Y, Bottlaender M, Aigrot M-S, Dolle F, Wu C, et al. Imaging of CNS myelin by positron-emission tomography. Proc Natl Acad Sci. 2006;103:9304–9.
Wang Y, Wu C, Caprariello AV, Somoza E, Zhu W, Wang C, et al. In vivo quantification of myelin changes in the vertebrate nervous system. J Neurosci. 2009;29:14663–9.
de Paula FD, Copray S, Sijbesma JWA, Willemsen ATM, Buchpiguel CA, Dierckx RAJO, et al. PET imaging of focal demyelination and remyelination in a rat model of multiple sclerosis: comparison of [11C]MeDAS, [11C]CIC and [11C]PIB. Eur J Nucl Med Mol Imaging. 2014;41:995–1003.
Wu C, Wang C, Popescu DC, Zhu W, Somoza EA, Zhu J, et al. A novel PET marker for in vivo quantification of myelination. Bioorg Med Chem. 2010;18:8592–9.
Wu C, Zhu J, Baeslack J, Zaremba A, Hecker J, Kraso J, et al. Longitudinal positron emission tomography imaging for monitoring myelin repair in the spinal cord. Ann Neurol. 2013;74:688–98.
van der Weijden CWJ, Meilof JF, van der Hoorn A, Zhu J, Wu C, Wang Y, et al. Quantitative assessment of myelin density using [11C]MeDAS PET in patients with multiple sclerosis: a first-in-human study. Eur J Nucl Med Mol Imaging. 2022;49:3492–507.
Fodero-Tavoletti MT, Rowe CC, McLean CA, Leone L, Li Q-X, Masters CL, et al. Characterization of PiB binding to white matter in Alzheimer Disease and other dementias. J Nucl Med. 2009;50:198–204.
Stankoff B, Freeman L, Aigrot M-S, Chardain A, Dollé F, Williams A, et al. Imaging central nervous system myelin by positron emission tomography in multiple sclerosis using [methyl-11C]-2-(4′-methylaminophenyl)- 6-hydroxybenzothiazole. Ann Neurol. 2011;69:673–80.
Carvalho RHF, Real CC, Cinini S, Garcez AT, Duran FLS, Marques FLN, et al. [11C]PIB PET imaging can detect white and grey matter demyelination in a non-human primate model of progressive multiple sclerosis. Mult Scler Relat Disord. 2019;35:108–15.
Bodini B, Veronese M, García-Lorenzo D, Battaglini M, Poirion E, Chardain A, et al. Dynamic imaging of individual remyelination profiles in multiple sclerosis. Ann Neurol. 2016;79:726–38.
Matías-Guiu JA, Cabrera-Martín MN, Matías-Guiu J, Oreja-Guevara C, Riola-Parada C, Moreno-Ramos T, et al. Amyloid PET imaging in multiple sclerosis: an 18F-florbetaben study. BMC Neurol. 2015;15:243.
Brugarolas P, Sánchez-Rodríguez JE, Tsai H-M, Basuli F, Cheng S-H, Zhang X, et al. Development of a PET radioligand for potassium channels to image CNS demyelination. Sci Rep. 2018;8:607.
Guehl NJ, Ramos-Torres KM, Linnman C, Moon S-H, Dhaynaut M, Wilks MQ, et al. Evaluation of the potassium channel tracer [18F]3F4AP in rhesus macaques. J Cereb Blood Flow Metab. 2021;41:1721–33.
Zivadinov R, Ramasamy DP, Hagemeier J, Kolb C, Bergsland N, Schweser F, et al. Evaluation of leptomeningeal contrast enhancement using pre-and postcontrast subtraction 3D-FLAIR imaging in multiple sclerosis. Am J Neuroradiol. 2018;39:642–7.
Kearney H, Miller DH, Ciccarelli O. Spinal cord MRI in multiple sclerosis—diagnostic, prognostic and clinical value. Nat Rev Neurol. 2015;11:327–38.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
MRB has received consulting fees from Genentech, EMD Serono, and Bristol Meyers Squibb and has received research support from the NIH and CMSC. FT has not conflicts to report.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brier, M.R., Taha, F. Measuring Pathology in Patients with Multiple Sclerosis Using Positron Emission Tomography. Curr Neurol Neurosci Rep 23, 479–488 (2023). https://doi.org/10.1007/s11910-023-01285-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-023-01285-z