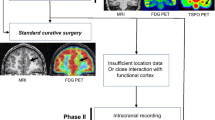
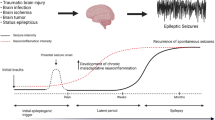
Abstract
Purpose of Review
Recent evidence indicates that chronic, low-level neuroinflammation underlies epileptogenesis. Targeted imaging of key neuroinflammatory cells, receptors, and tissues may enable localizing epileptogenic onset zone, especially in those patients who are treatment-resistant and considered MRI-negative. Finding a specific, sensitive neuroimaging-based biomarker could aid surgical planning and improve overall prognosis in eligible patients. This article reviews recent research on in vivo imaging of neuroinflammatory targets in patients with treatment-resistant, non-lesional epilepsy.
Recent Findings
A number of advanced approaches based on imaging neuroinflammation are being implemented in order to assist localization of epileptogenic onset zone. The most exciting tools are based on radioligand-based nuclear imaging or revisiting of existing technology in novel ways. The greatest limitations stem from gaps in knowledge about the exact function of neuroinflammatory targets (e.g., neurotoxic or neuroprotective). Further, lingering questions about each approach’s specificity, reliability, and sensitivity must be addressed, and clinical utility must be validated before any novel method is incorporated into mainstream clinical practice.
Summary
Current applications of imaging neuroinflammation in humans are limited and underutilized, but offer hope for finding sensitive and specific neuroimaging-based biomarker(s). Future work necessitates appreciation of investigations to date, significant findings, and neuroinflammatory targets worth exploring further.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs a 30-year longitudinal cohort study. JAMA Neurol. 2018;75(3):279–86.
Laxer KD, Trinka E, Hirsch LJ, Cendes F, Langfitt J, Delanty N, et al. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014;37:59–70.
Kent GP, Schefft BK, Howe SR, Szaflarski JP, Yeh HS, Privitera MD. The effects of duration of intractable epilepsy on memory function. Epilepsy Behav. 2006;9(3):469–77.
Black LC, Schefft BK, Howe SR, Szaflarski JP, Shain YH, Privitera MD. The effect of seizures on working memory and executive functioning performance. Epilepsy Behav. 2010;17:412–9.
Vannest J, Szaflarski JP, Privitera MD, Schefft BK, Holland SK. Medial temporal fMRI activation reflects memory lateralization and memory performance in patients with epilepsy. Epilepsy Behav. 2008;12(3):410–8.
Lhatoo SD, Johnson AL, Goodridge DM, MacDonald BK, Sander JWAS, Shorvon SD. Mortality in epilepsy in the first 11 to 14 years after diagnosis: multivariate analysis of a long-term, prospective, population-based cohort. Ann Neurol. 2001;49(3):336–44.
Sillanpää M, Shinnar S. SUDEP and other causes of mortality in childhood-onset epilepsy. Epilepsy Behav. 2013;28(2):249–55.
Derby LE, Tennis P, Jick H. Sudden unexplained death among subjects with refractory epilepsy. Epilepsia. 1996;37(10):931–5.
Walczak TS, Leppik IE, D’Amelio M, Rarick J, So E, Ahman P, et al. Incidence and risk factors in sudden unexpected death in epilepsy. Neurology. 2001;56(4):519–25.
Szaflarski JP, Szaflarski M. Seizure disorders, depression, and health-related quality of life. Epilepsy Behav. 2004;5(1):50–7.
Noe K, Sulc V, Wong-Kisiel L, Wirrell E, Van Gompel JJ, Wetjen N, et al. Long-term outcomes after nonlesional extratemporal lobe epilepsy surgery. JAMA Neurol. 2013;70(8):1003–8.
Jehi L. The epileptogenic zone: concept and definition. Epilepsy Curr. 2018;18:12–6.
Salmenpera M, Symms MR, Rugg-Gunn FJ, Boulby PA, Free SL, Barker J, et al. Evaluation of quantitative magnetic resonance imaging contrasts in MRI-negative refractory focal epilepsy. Epilepsia. 2007;48(2):229–37.
Jobst BC, Cascino GD. Resective epilepsy surgery for drug-resistant focal epilepsy: a review. JAMA. 2015;313(3):285–93.
Téllez-Zenteno JF, Ronquillo LH, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res. 2010;89(2–3):310–8.
Middlebrooks EH, Ver Hoef L, Szaflarski JP. Neuroimaging in epilepsy. Curr Neurol Neurosci Rep. 2017;17(4):32.
Urbach H, Binder D, von Lehe M, Podlogar M, Bien CG, Becker A, et al. Correlation of MRI and histopathology in epileptogenic parietal and occipital lobe lesions. Seizure. 2007;16(7):608–14.
Hakami T, McIntosh A, Todaro M, Lui E, Yerra R, Tan KM, et al. MRI-identified pathology in adults with new-onset seizures. Neurology. 2013;81(10):920–7.
• Wang S, Jin B, Aung T, Katagiri M, Jones SE, Krishnan B, et al. Application of MRI post-processing in presurgical evaluation of non-lesional cingulate epilepsy. Front Neurol. 2018;9:1–7 The authors applied an MRI post-processing technique called MAP that allowed visualizing lesions in sMRI-negative non-lesional epilepsy patients.
Wang ZI, Jones SE, Jaisani Z, Najm IM, Prayson RA, Burgess RC, et al. Voxel-based morphometric magnetic resonance imaging (MRI) postprocessing in MRI-negative epilepsies. Ann Neurol. 2015;77(6):1060–75.
Gaillard WD, Cross JH, Duncan JS, Stefan H, Theodore WH. Epilepsy imaging study guideline criteria: commentary on diagnostic testing study guidelines and practice parameters. Epilepsia. 2011;52(9):1750–6.
Muhlhofer W, Tan Y-L, Mueller SG, Knowlton R. MRI-negative temporal lobe epilepsy-what do we know? Epilepsia. 2017;58(5):727–42.
Vezzani A, Balosso S, Ravizza T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol. 2019;15(8):459–72.
Vezzani A, Friedman A, Dingledine RJ. The role of inflammation in epileptogenesis. Neuropharmacology. 2013;69:16–24.
Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16(4):413–9.
Vezzani A, Friedman A. Brain inflammation as a biomarker in epilepsy. Biomark Med. 2011;5(5):607–14.
Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7(1):31–40.
Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229(2):176–85.
Therajaran P, Hamilton JA, O’Brien TJ, Jones NC, Ali I. Microglial polarization in posttraumatic epilepsy: potential mechanism and treatment opportunity. Epilepsia. 2020;00:1–23.
Corps KN, Roth TL, McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015;72(3):355–62.
Ravizza T, Gagliardi B, Noé F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008;29(1):142–60.
Ravizza T, Balosso S, Vezzani A. Inflammation and prevention of epileptogenesis. Neurosci Lett. 2011;497(3):223–30.
Balosso S, Ravizza T, Pierucci M, Calcagno E, Invernizzi R, Di Giovanni G, et al. Molecular and functional interactions between tumor necrosis factor-alpha receptors and the glutamatergic system in the mouse hippocampus: implications for seizure susceptibility. Neuroscience. 2009;161(1):293–300.
Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA. Glia and epilepsy: excitability and inflammation. Trends Neurosci. 2013;36(3):174–84.
Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11(6):805–21.
Dahnke R, Yotter RA, Gaser C. Cortical thickness and central surface estimation. Neuroimage. 2013;65:336–48.
Malinska D, Kulawiak B, Kudin AP, Kovacs R, Huchzermeyer C, Kann O, et al. Complex III-dependent superoxide production of brain mitochondria contributes to seizure-related ROS formation. Biochim Biophys Acta Bioenerg. 2010;1797(6–7):1163–70.
Blümcke I, Coras R, Miyata H, Özkara C. Defining clinico-neuropathological subtypes of mesial temporal lobe epilepsy with hippocampal sclerosis. Brain Pathol. 2012;22(3):402–11.
Kälviäinen R, Salmenperä T. Do recurrent seizures cause neuronal damage? A series of studies with MRI volumetry in adults with partial epilepsy. Prog Brain Res. 2002;135:279–95.
Barr WB, Ashtari M, Schaul N. Bilateral reductions in hippocampal volume in adults with epilepsy and a history of febrile seizures. J Neurol Neurosurg Psychiatry. 1997;63(4):461–7.
Buonocore MH, Maddock RJ. Magnetic resonance spectroscopy of the brain: a review of physical principles and technical methods. Rev Neurosci. 2015;26(6):609–32.
Oz G, Alger JR, Barker PB, Bartha R, Bizzi A, Boesch C, et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology. 2014;270(3):658–79.
Arnold DL, De Stefano N. Magnetic resonance spectroscopy in vivo: applications in neurological disorders. Ital J Neurol Sci. 1997;18(6):321–9.
Maudsley AA, Darkazanli A, Alger JR, Hall LO, Schuff N, Studholme C, et al. Comprehensive processing, display and analysis for in vivo MR spectroscopic imaging. NMR Biomed. 2006;19(4):492–503.
Bertholdo D, Watcharakorn A, Castillo M. Brain proton magnetic resonance spectroscopy: introduction and overview. Neuroimaging Clin N Am. 2013;23:359–80.
• Kirov II, Kuzniecky R, Hetherington HP, Soher BJ, Davitz MS, Babb JS, et al. Whole brain neuronal abnormalities in focal quantified with proton MR spectroscopy. Epilepsy Res. 2018;139:85–91 First human study that used whole-brain1H-MRS demonstrates that epilepsy may be a network disorder, as based on widespread, diffuse neuronal dysfunction and global NAA reductions extending well beyond the epileptogenic zone.
Simister RJ, Woermann FG, McLean MA, Bartlett PA, Barker GJ, Duncan JS. A short-echo-time proton magnetic resonance spectroscopic imaging study of temporal lobe epilepsy. Epilepsia. 2002;43(9):1021–31.
Simister RJ, McLean MA, Barker GJ, Duncan JS. Proton MRS reveals frontal lobe metabolite abnormalities in idiopathic generalized epilepsy. Neurology. 2003;61(7):897–902.
Simister RJ, McLean MA, Barker GJ, Duncan JS. Proton magnetic resonance spectroscopy of malformations of cortical development causing epilepsy. Epilepsy Res. 2007;74(2–3):107–15.
Simister RJ, McLean MA, Barker GJ, Duncan JS. The effect of sodium valproate on proton MRS visible neurochemical concentrations. Epilepsy Res. 2007;74(2–3):215–9.
Simister RJ, McLean MA, Barker GJ, Duncan JS. A proton magnetic resonance spectroscopy study of metabolites in the occipital lobes in epilepsy. Epilepsia. 2003;44(4):550–8.
Petroff OA, Hyder F, Rothman DL, Mattson RH. Homocarnosine and seizure control in juvenile myoclonic epilepsy and complex partial seizures. Neurology. 2001;56(6):709–15.
Rincon SP, Blitstein MBK, Caruso PA, González RG, Thibert RL, Ratai E-M. The use of magnetic resonance spectroscopy in the evaluation of pediatric patients with seizures. Pediatr Neurol. 2016;58:57–66.
Ladd ME, Bachert P, Meyerspeer M, Moser E, Nagel AM, Norris DG, et al. Pros and cons of ultra-high-field MRI/MRS for human application. Prog Nucl Magn Reson Spectrosc. 2018;109:1–50.
van der Zijden JP, van Eijsden P, de Graaf RA, Dijkhuizen RM. 1H/13C MR spectroscopic imaging of regionally specific metabolic alterations after experimental stroke. Brain. 2008;131(8):2209–19.
Pan JW, Kuzniecky RI. Utility of magnetic resonance spectroscopic imaging for human epilepsy. Quant Imaging Med Surg. 2015;5(2):313–22.
Wang H, Wang B, Normoyle KP, Jackson K, Spitler K, Sharrock M, et al. Brain temperature and its fundamental properties: a review for clinical neuroscientists. Front Neurosci. 2014;8(307):1–17.
•• Mueller C, Lin JC, Sheriff S, Maudsley AA, Younger JW. Evidence of widespread metabolite abnormalities in myalgic encephalomyelitis/chronic fatigue syndrome: assessment with whole-brain magnetic resonance spectroscopy. Brain Imaging Behav. 2019; The authors used whole-brain MRS to study abnormal neuroinflammatory markers, notably brain temperature increases, in 15 patients with myalgic encephalomyelitis/chronic fatigue syndrome and 15 healthy controls. This is one of the first groups to apply MRS-t approach to brain temperature mapping in a patient population.
Rieke V, Butts PK. MR thermometry. J Magn Reson Imaging. 2008;27(2):376–90.
McCracken DJ, Willie JT, Fernald BA, Saindane AM, Drane DL, Barrow DL, et al. Magnetic resonance thermometry-guided stereotactic laser ablation of cavernous malformations in drug-resistant epilepsy. Oper Neurosurg. 2016;12(1):39–48.
Hirashima Y, Takaba M, Endo S, Hayashi N, Yamashita K, Takaku A. Intracerebral temperature in patients with hydrocephalus of varying aetiology. J Neurol Neurosurg Psychiatry. 1998;64(6):792–4.
Rossi S, Roncati Zanier E, Mauri I, Columbo A, Stocchetti N. Brain temperature, body core temperature, and intracranial pressure in acute cerebral damage. J Neurol Neurosurg Psychiatry. 2001;71(4):448–54.
Orihuela R, McPherson CA, Harry GJ. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol. 2016;173(4):649–65.
•• Maudsley AA, Goryawala MZ, Sheriff S. Effects of tissue susceptibility on brain temperature mapping. Neuroimage. 2017;146:1093–101 This study proposed a novel approach for non-invasive brain temperature measurement using whole-brain MRS, with guidelines for data acquisition and image processing, as well as thorough consideration of tissue susceptibility variations that limit the interpretation of results. This study also established Cre as the reference metabolite of choice for brain temperature mapping.
Dehkharghani S, Mao H, Howell L, Zhang X, Pate KS, Magrath PR, et al. Proton resonance frequency chemical shift thermometry: experimental design and validation toward high-resolution noninvasive temperature monitoring and in vivo experience in a nonhuman primate model of acute ischemic stroke. AJNR Am J Neuroradiol. 2015;36(6):1128–35.
Maudsley AA, Domenig C, Sheriff S. Reproducibility of serial whole-brain MR spectroscopic imaging. NMR Biomed. 2010 Apr;23(3):251–6.
Sharma A, Nenert R, Mueller C, Maudsley A, Younger J, Szaflarski J. Test-retest reproducibility of brain temperature derivations using echoplanar spectroscopic imaging. Abstract submitted to 2020 Organization for Human Brain Mapping Conference. June 25–29, 2020. Montreal.
Kuhl DE, Engel J, Phelps ME, Selin C. Epileptic patterns of local cerebral metabolism and perfusion in humans determined by emission computed tomography of 18FDG and 13NH3. Ann Neurol. 1980;8(4):348–60.
Chugani DC, Muzik O. α[C-11]methyl-L-tryptophan PET maps brain serotonin synthesis and Kynurenine pathway metabolism. J Cereb Blood Flow Metab. 2000;20:2–9.
Chugani DC. α-Methyl-L-tryptophan: mechanisms for tracer localization of epileptogenic brain regions. Biomark Med. 2011;5:567–75.
Chugani HT, Luat AF, Kumar A, Govindan R, Pawlik K, Asano E. α-[11C]-Methyl-L-tryptophan-PET in 191 patients with tuberous sclerosis complex. Neurology. 2013;81(7):674–80.
Fedi M, Reutens D, Okazawa H, Andermann F, Boling W, Dubeau F, et al. Localizing value of α-methyl-L-tryptophan PET in intractable epilepsy of neocortical origin. Neurology. 2001;57(9):1629–36.
Willmann O, Wennberg R, May T, Woermann FG, Pohlmann-Eden B. The contribution of 18F-FDG PET in preoperative epilepsy surgery evaluation for patients with temporal lobe epilepsy. A meta-analysis. Seizure. 2007;16(6):509–20.
O’Brien TJ, Newton MR, Cook MJ, Berlangieri SU, Kilpatrick C, Morris K, et al. Hippocampal atrophy is not a major determinant of regional hypometabolism in temporal lobe epilepsy. Epilepsia. 1997;38(1):74–80.
Lamusuo S, Jutila L, Ylinen A, Kälviäinen R, Mervaala E, Haaparanta M, et al. [18F]FDG-PET reveals temporal hypometabolism in patients with temporal lobe epilepsy even when quantitative MRI and histopathological analysis show only mild hippocampal damage. Arch Neurol. 2001;58(6):933–9.
Henry TR, Babb TL, Engel J, Mazziotta JC, Phelps ME, Crandall PH. Hippocampal neuronal loss and regional hypometabolism in temporal lobe epilepsy. Ann Neurol. 1994;36(6):925–7.
Probasco JC, Solnes L, Nalluri A, Cohen J, Jones KM, Zan E, et al. Abnormal brain metabolism on FDG-PET/CT is a common early finding in autoimmune encephalitis. Neurol Neuroimmunol NeuroInflammation. 2017;4.
Schur S, Allen V, White A, Mirsky D, Stence N, O’Neill B, et al. Significance of FDG-PET hypermetabolism in children with intractable focal epilepsy. Pediatr Neurosurg. 2018;53(3):153–62.
Gaspard N, Foreman BP, Alvarez V, Kang CC, Probasco JC, Jongeling AC, et al. New-onset refractory status epilepticus. Neurology. 2015;85(18):1604–13.
Khawaja AM, DeWolfe JL, Miller DW, Szaflarski JP. New-onset refractory status epilepticus (NORSE)-the potential role for immunotherapy. Epilepsy Behav. 2015;47:17–23.
Khawaja AM, Vines BL, Miller DW, Szaflarski JP, Amara AW. Refractory status epilepticus and glutamic acid decarboxylase antibodies in adults: presentation, treatment and outcomes. Epileptic Disord. 2016;18(1):34–43.
Choi JY, Kim SJ, Hong SB, Seo DW, Hong SC, Kim BT, et al. Extratemporal hypometabolism on FDG PET in temporal lobe epilepsy as a predictor of seizure outcome after temporal lobectomy. Eur J Nucl Med Mol Imaging. 2003;30(4):581–7.
Koutroumanidis M, Binnie CD, Elwes RDC, Polkey CE, Seed P, Alarcon G, et al. Interictal regional slow activity in temporal lobe epilepsy correlates with lateral temporal hypometabolism as imaged with 18 FDG PET: neurophysiological and metabolic implications. J Neurol Neurosurg Psychiatry. 1998;65(2):170–6.
Dupont S, Semah F, Clémenceau S, Adam C, Baulac M, Samson Y. Accurate prediction of postoperative outcome in mesial temporal lobe epilepsy: a study using positron emission tomography with 18fluorodeoxyglucose. Arch Neurol. 2000;57(9):1331–6.
Radtke RA, Hanson MW, Hoffman JM, Crain BJ, Walczak TS, Lewis DV, et al. Temporal lobe hypometabolism on pet: predictor of seizure control after temporal lobectomy. Neurology. 1993;43(6):1088–92.
Tenney JR, Rozhkov L, Horn P, Miles L, Miles MV. Cerebral glucose hypometabolism is associated with mitochondrial dysfunction in patients with intractable epilepsy and cortical dysplasia. Epilepsia. 2014;55(9):1415–22.
Koenig JB, Dulla CG. Dysregulated glucose metabolism as a therapeutic target to reduce post-traumatic epilepsy. Front Cell Neurosci. 2018;12.
Owen DR, Guo Q, Kalk NJ, Colasanti A, Kalogiannopoulou D, Dimber R, et al. Determination of [11C]PBR28 binding potential in vivo: a first human TSPO blocking study. J Cereb Blood Flow Metab. 2014;34(6):989–94.
Vivash L, OBrien TJ. Imaging microglial activation with TSPO PET: lighting up neurologic diseases? J Nucl Med. 2016;57(2):165–8.
Banati RB, Goerres GW, Myers R, Gunn RN, Turkheimer FE, Kreutzberg GW, et al. [11C](R)-PK11195 positron emission tomography imaging of activated microglia in vivo in Rasmussen’s encephalitis. Neurology. 1999;53(9):2199–203.
Butler T, Ichise M, Teich AF, Gerard E, Osborne J, French J, et al. Imaging inflammation in a patient with epilepsy due to focal cortical dysplasia. J Neuroimaging. 2013;23(1):129–311.
Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab. 2012;32(1):1–5.
Sharma AA, Szaflarski JP. Seizing the neuroinflammatory target: the quest continues. Epilepsy Curr. 2019;19(6):379–81.
Gershen LD, Zanotti-Fregonara P, Dustin IH, Liow J-S, Hirvonen J, Kreisl WC, et al. Neuroinflammation in temporal lobe epilepsy measured using positron emission tomographic imaging of translocator protein. JAMA Neurol. 2015;72(8):882–8.
Hirvonen J, Kreisl WC, Fujita M, Dustin I, Khan O, Appel S, et al. Increased in vivo expression of an inflammatory marker in temporal lobe epilepsy. J Nucl Med. 2012;53(2):234–40.
• Dickstein LP, Liow J, Austermuehle A, Zoghbi S, Inati SK, Zaghloul K, et al. Neuroinflammation in neocortical epilepsy measured by PET imaging of translocator protein. Epilepsia. 2019;60(6) This is the first human study to demonstrate that TSPO-based PET may be a promising approach for delineating ictal onset zone in non-lesional epilepsy, as based on evaluation of 9 patients with neocortical epilepsy and 11 age-matched healthy controls following genetic stratification for TSPO-binding affinity.
Cosenza-Nashat M, Zhao M-L, Suh H-S, Morgan J, Natividad R, Morgello S, et al. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol. 2009;35(3):306–28.
Tang Y, Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol. 2016;53(2):1181–94.
Ghadery C, Best LA, Pavese N, Tai YF, Strafella AP. PET evaluation of microglial activation in non-neurodegenerative brain diseases. Curr Neurol Neurosci Rep. 2019;19(7):38.
Kasantikul V, Brown WJ, Oldendorf WH, Crandall PC. Ultrastructural parameters of limbic microvasculature in human psychomotor epilepsy. Clin Neuropathol. 1983;2(4):171–8.
Cornford EM, Hyman S, Cornford ME, Landaw EM, Delgado-Escueta AV. Interictal seizure resections show two configurations of endothelial Glut1 glucose transporter in the human blood-brain barrier. J Cereb Blood Flow Metab. 1998;18(1):26–42.
Fieschi C, Lenzi GL, Zanette E, Orzi F, Passero S. Effects of EEG of the osmotic opening of the blood-brain barrier in rats. Life Sci. 1980;27(3):239–43.
•• Marchi N, Angelov L, Masaryk T, Fazio V, Granata T, Hernandez N, et al. Seizure-promoting effect of blood-brain barrier disruption. Epilepsia. 2007;48(4):732–42 First study in humans to demonstrate the seizure-promoting effect of acute BBB disruption; following acute osmotic disruption of the BBB, 25% of brain lymphoma patients developed focal seizures in 25%.
Ku MC, Waiczies S, Niendorf T, Pohlmann A. Assessment of blood brain barrier leakage with gadolinium-enhanced MRI. Methods Mol Bio. 1718;2018:395–408.
Han H, Mann A, Ekstein D, Eyal S. Breaking bad: the structure and function of the blood-brain barrier in epilepsy. AAPS J. 2017;19(4):973–88.
Tomkins O, Shelef I, Kaizerman I, Eliushin A, Afawi Z, Misk A, Gidon M, Cohen A, Zumsteg D, Friedman A. Blood-brain barrier disruption in post-traumatic epilepsy. J Neurol Neurosurg Psychiatry 2008;79(7):774–7.
Cascino GD, Hirschorn KA, Jack CR, Sharbrough FW. Gadolinium-DTPA-enhanced magnetic resonance imaging in intractable partial epilepsy. Neurology. 1989;39(8):1115–8.
Portnoy E, Polyak B, Inbar D, Kenan G, Rai A, Wehrli SL, et al. Tracking inflammation in the epileptic rat brain by bi-functional fluorescent and magnetic nanoparticles. Nanomedicine. 2016;12(5):1335–45.
Duffy BA, Choy MK, Riegler J, Wells JA, Anthony DC, Scott RC, et al. Imaging seizure-induced inflammation using an antibody targeted iron oxide contrast agent. Neuroimage. 2012;60(2):1149–55.
Tishler DM, Weinberg KI, Hinton DR, Barbaro N, Annett GM, Raffel C. MDR1 gene expression in brain of patients with medically intractable epilepsy. Epilepsia. 1995;36(1):1–6.
Stȩpień KM, Tomaszewski M, Tomaszewska J, Czuczwar SJ. The multidrug transporter P-glycoprotein in pharmacoresistance to antiepileptic drugs. Pharmacol Rep. 2012;64:1011–109.
Potschka H. Role of CNS efflux drug transporters in antiepileptic drug delivery: overcoming CNS efflux drug transport. Adv Drug Deliv Rev. 2012;64(10):943–52.
Löscher W. Drug transporters in the epileptic brain. In: Epilepsia; 2007. p. 8–13.
Luurtsema G, Molthoff CFM, Schuit RC, Windhorst AD, Lammertsma AA, Franssen EJF. Evaluation of (R)-[11C]verapamil as PET tracer of P-glycoprotein function in the blood-brain barrier: kinetics and metabolism in the rat. Nucl Med Biol. 2005;32(1):87–93.
Langer O, Bauer M, Hammers A, Karch R, Pataraia E, Koepp MJ, et al. Pharmacoresistance in epilepsy: a pilot PET study with the P-glycoprotein substrate R-[(11)C]verapamil. Epilepsia. 2007;48(9):1774–84.
Feldmann M, Asselin M-C, Liu J, Wang S, McMahon A, Anton-Rodriguez J, et al. P-glycoprotein expression and function in patients with temporal lobe epilepsy: a case-control study. Lancet Neurol. 2013;12(8):777–85.
Shin JW, Chu K, Shin SA, Jung KH, Lee ST, Lee YS, et al. Clinical applications of simultaneous PET/MR imaging using (R)-[11C]-verapamil with cyclosporin A: preliminary results on a surrogate marker of drug-resistant epilepsy. Am J Neuroradiol. 2016;37:600–6.
Rizzo G, Veronese M, Tonietto M, Zanotti-Fregonara P, Turkheimer FE, Bertoldo A. Kinetic modeling without accounting for the vascular component impairs the quantification of [11C]PBR28 brain PET data. J Cereb Blood Flow Metab. 2014;34(6):1060–9.
Pan JW, Williamson A, Cavus I, Hetherington HP, Zaveri H, Petroff OAC, et al. Neurometabolism in human epilepsy. Epilepsia. 2008;49:31–41.
Cavus I, Pan JW, Hetherington HP, Abi-Saab W, Zaveri HP, Vives KP, et al. Decreased hippocampal volume on MRI is associated with increased extracellular glutamate in epilepsy patients. Epilepsia. 2008;49(8):1358–66.
Pfund Z, Chugani DC, Juhász C, Muzik O, Chugani HT, Wilds IB, et al. Evidence for coupling between glucose metabolism and glutamate cycling using FDG PET and1H magnetic resonance spectroscopy in patients with epilepsy. J Cereb Blood Flow Metab. 2000;20(5):871–8.
Eid T, Thomas MJ, Spencer DD, Rundén-Pran E, Lai JCK, Malthankar GV, et al. Loss of glutamine synthetase in the human epileptogenic hippocampus: possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet (London, England). 2004;363(9402):28–37.
Ramadan S, Lin A, Stanwell P. Glutamate and glutamine: a review of in vivo MRS in the human brain. NMR Biomed. 2013;26:1630–46.
Davis KA, Nanga RPR, Das S, Chen SH, Hadar PN, Pollard JR, et al. Glutamate imaging (GluCEST) lateralizes epileptic foci in nonlesional temporal lobe epilepsy. Sci Transl Med. 2015;7(309).
Cai K, Haris M, Singh A, Kogan F, Greenberg JH, Hariharan H, et al. Magnetic resonance imaging of glutamate. Nat Med. 2012;18(2):302–6.
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;15:4(147).
Jessen NA, Munk ASF, Lundgaard I, Nedergaard M. The glymphatic system: a beginner’s guide. Neurochem Res. 2015;40(12):2583–99.
Plog BA, Dashnaw ML, Hitomi E, Peng W, Liao Y, Lou N, et al. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci. 2015;35(2):518–26.
Golanov EV, Bovshik EI, Wong KK, Pautler RG, Foster CH, Federley RG, et al. Subarachnoid hemorrhage–induced block of cerebrospinal fluid flow: role of brain coagulation factor III (tissue factor). J Cereb Blood Flow Metab. 2018;38(5):793–808.
Goulay R, Flament J, Gauberti M, Naveau M, Pasquet N, Gakuba C, et al. Subarachnoid hemorrhage severely impairs brain parenchymal cerebrospinal fluid circulation in nonhuman primate. Stroke. 2017;48(8):2301–5.
Gaberel T, Gakuba C, Goulay R, De Lizarrondo SM, Hanouz JL, Emery E, et al. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: a new target for fibrinolysis? Stroke. 2014;45(10):3092–6.
De Leon MJ, Li Y, Okamura N, Tsui WH, Saint-Louis LA, Glodzik L, et al. Cerebrospinal fluid clearance in Alzheimer disease measured with dynamic PET. J Nucl Med. 2017;58(9):1471–6.
•• Taoka T, Masutani Y, Kawai H, Nakane T, Matsuoka K, Yasuno F, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J Radiol. 2017;35(4):172–8 The authors established the feasibility of non-invasively assessing glymphatic function using DTI-ALPS in the context of Alzheimer’s disease. The study’s methodology and conceptual basis are applicable to studying epilepsy.
Helakari H, Kananen J, Huotari N, Raitamaa L, Tuovinen T, Borchardt V, et al. Spectral entropy indicates electrophysiological and hemodynamic changes in drug-resistant epilepsy–a multimodal MREG study. NeuroImage Clin. 2019;22.
•• Kiviniemi V, Wang X, Korhonen V, Keinänen T, Tuovinen T, Autio J, et al. Ultra-fast magnetic resonance encephalography of physiological brain activity-glymphatic pulsation mechanisms? J Cereb Blood Flow Metab. 2016;36(6):1033–45 The authors used simultaneous MREG BOLD with continuous ECG and non-invasive blood pressure measurements to indirectly assess glymphatic function on the basis of cardiac, respiratory, and low-frequency pulsations driving CSF fluid dynamics.
Eide PK, Ringstad G. MRI with intrathecal MRI gadolinium contrast medium administration: a possible method to assess glymphatic function in human brain. Acta Radiol Open. 2015;4(11).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ayushe Alicia Sharma and Jerzy P. Szaflarski each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Epilepsy
Rights and permissions
About this article
Cite this article
Sharma, A.A., Szaflarski, J.P. In Vivo Imaging of Neuroinflammatory Targets in Treatment-Resistant Epilepsy. Curr Neurol Neurosci Rep 20, 5 (2020). https://doi.org/10.1007/s11910-020-1025-9
Published:
DOI: https://doi.org/10.1007/s11910-020-1025-9