Abstract
Purpose of Review
This article strives to review and summarize selected recent literature and topics contributing to a greater understanding of the diagnosis and treatments of neonatal seizures that have emerged in the past several years.
Recent Findings
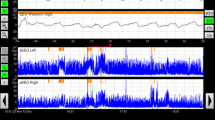
Continuous EEG is recommended as the gold standard for neonatal seizure monitoring as it can provide additional information that may stratify patients by etiology, as well as identify at-risk groups of newborns for neuromonitoring. Investigations are moving beyond traditional antiepileptic agents in search of treatments with better efficacy and with less concern for developmental effects. Targeted therapies for seizures resulting from particular genetic conditions are increasing, highlighting the importance of early genetic diagnosis. Better understanding of the risk of post-neonatal epilepsy based on etiology is emerging with new epidemiological studies.
Summary
Evidence is growing for deleterious effects of seizures on outcomes, elevating the importance of seizure detection and effective treatment. Advances in utilization of continuous EEG monitoring have improved the accuracy of seizure detection and have identified at-risk groups of newborns for neuromonitoring. Ultimately, the goal in management of neonatal seizures is not only clinical stabilization in the acute period but also to influence neurodevelopmental outcome and modify the risk of future epilepsy.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Vasudevan C, Levene M. Epidemiology and aetiology of neonatal seizures. Semin Fetal Neonatal Med. 2013;18:185–91.
Wilmshurst JM, Gaillard WD, Vinayan KP, et al. Summary of recommendations for the management of infantile seizures: Task Force Report for the ILAE Commission of Pediatrics. Epilepsia. 2015;56:1185–97.
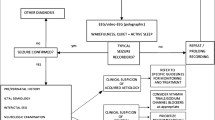
Loman AM, ter Horst HJ, Lambrechtsen FA, Lunsing RJ. Neonatal seizures: aetiology by means of a standardized work-up. Eur J Paediatr Neurol. 2014;18:360–7.
Donovan MD, Griffin BT, Kharoshankaya L, Cryan JF, Boylan GB. Pharmacotherapy for neonatal seizures: current knowledge and future perspectives. Drugs. 2016;76(6):647–61.
Tymofiyeva O, Hess CP, Xu D, Barkovich AJ. Structural MRI connectome in development: challenges of the changing brain. Br J Radiol. 2014;87:20140086.
Briggs SW, Galanopoulou AS. Altered GABA signaling in early life epilepsies. Neural Plast. 2011;2011:527605.
Murray DM, Boylan GB, Ali I, Ryan CA, Murphy BP, Connolly S. Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Arch Dis Child Fetal Neonatal Ed. 2008;93:F187–191.
Nash KB, Bonifacio SL, Glass HC, et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neurology. 2011;76:556–62.
Maartens IA, Wassenberg T, Buijs J, et al. Neurodevelopmental outcome in full-term newborns with refractory neonatal seizures. Acta Paediatr. 2012;101:e173–178.
Pisani F, Cerminara C, Fusco C, Sisti L. Neonatal status epilepticus vs recurrent neonatal seizures: clinical findings and outcome. Neurology. 2007;69:2177–85.
Srinivasakumar P, Zempel J, Trivedi S, et al. Treating EEG seizures in hypoxic ischemic encephalopathy: a randomized controlled trial. Pediatrics. 2015;136:e1302–1309.
Shetty J. Neonatal seizures in hypoxic-ischaemic encephalopathy—risks and benefits of anticonvulsant therapy. Dev Med Child Neurol. 2015;57 Suppl 3:40–3.
Bittigau P, Sifringer M, Genz K, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci U S A. 2002;99:15089–94.
Ikonomidou C. Triggers of apoptosis in the immature brain. Brain Dev. 2009;31:488–92.
Shellhaas RA, Chang T, Tsuchida T, et al. The American Clinical Neurophysiology Society’s Guideline on continuous electroencephalography monitoring in neonates. J Clin Neurophysiol. 2011;28:611–7.
Pisani F, Facini C, Pavlidis E, Spagnoli C, Boylan G. Epilepsy after neonatal seizures: literature review. Eur J Paediatr Neurol. 2015;19:6–14.
Rakshasbhuvankar A, Paul S, Nagarajan L, Ghosh S, Rao S. Amplitude-integrated EEG for detection of neonatal seizures: a systematic review. Seizure. 2015;33:90–8.
Shellhaas RA, Soaita AI, Clancy RR. Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics. 2007;120:770–7.
Boylan GB, Stevenson NJ, Vanhatalo S. Monitoring neonatal seizures. Semin Fetal Neonatal Med. 2013;18:202–8.
Wietstock SO, Bonifacio SL, Sullivan JE, Nash KB, Glass HC. Continuous video electroencephalographic (EEG) monitoring for electrographic seizure diagnosis in neonates: a single-center study. J Child Neurol. 2016;31:328–32.
Glass HC, Shellhaas RA, Wusthoff CJ, et al. Contemporary profile of seizures in neonates: a prospective cohort study. J Pediatr. 2016;174:98–103. e101.
Weeke LC, Boylan GB, Pressler RM, et al. Role of EEG background activity, seizure burden and MRI in predicting neurodevelopmental outcome in full-term infants with hypoxic-ischaemic encephalopathy in the era of therapeutic hypothermia. Eur J Paediatr Neurol 2016. doi:10.1016/j.ejpn.2016.06.003. This article highlights the role of EEG and MRI in prediction of outcome of patients with HIE who have undergone hypothermia, which may affect both testing modalities
Cnossen MH, van Ommen CH, Appel IM. Etiology and treatment of perinatal stroke; a role for prothrombotic coagulation factors? Semin Fetal Neonatal Med. 2009;14:311–7.
Cowan F, Mercuri E, Groenendaal F, et al. Does cranial ultrasound imaging identify arterial cerebral infarction in term neonates? Arch Dis Child Fetal Neonatal Ed. 2005;90:F252–256.
Martinez-Biarge M, Cheong JL, Diez-Sebastian J, Mercuri E, Dubowitz LM, Cowan FM. Risk factors for neonatal arterial ischemic stroke: the importance of the intrapartum period. J Pediatr. 2016;3:62–68.e1.
Low E, Mathieson SR, Stevenson NJ, et al. Early postnatal EEG features of perinatal arterial ischaemic stroke with seizures. PLoS One. 2014;9:e100973.
Naim MY, Gaynor JW, Chen J, et al. Subclinical seizures identified by postoperative electroencephalographic monitoring are common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2015;150:169–78. discussion 178-180.
Pisani F, Facini C, Pelosi A, Mazzotta S, Spagnoli C, Pavlidis E. Neonatal seizures in preterm newborns: a predictive model for outcome. Eur J Paediatr Neurol. 2016;20:243–51.
Sheth RD, Hobbs GR, Mullett M. Neonatal seizures: incidence, onset, and etiology by gestational age. J Perinatol. 1999;19:40–3.
Vesoulis ZA, Inder TE, Woodward LJ, Buse B, Vavasseur C, Mathur AM. Early electrographic seizures, brain injury, and neurodevelopmental risk in the very preterm infant. Pediatr Res. 2014;75:564–9.
Guidelines on Neonatal Seizures. Geneva: World Health Organization; 2011.
Painter MJ, Scher MS, Stein AD, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med. 1999;341:485–9.
van den Broek MP, van Straaten HL, Huitema AD, et al. Anticonvulsant effectiveness and hemodynamic safety of midazolam in full-term infants treated with hypothermia. Neonatology. 2015;107:150–6.
Boylan GB, Rennie JM, Chorley G, et al. Second-line anticonvulsant treatment of neonatal seizures: a video-EEG monitoring study. Neurology. 2004;62:486–8.
Silverstein FS, Ferriero DM. Off-label use of antiepileptic drugs for the treatment of neonatal seizures. Pediatr Neurol. 2008;39:77–9.
Lynch NE, Stevenson NJ, Livingstone V, Murphy BP, Rennie JM, Boylan GB. The temporal evolution of electrographic seizure burden in neonatal hypoxic ischemic encephalopathy. Epilepsia. 2012;53:549–57.
Edwards AD, Brocklehurst P, Gunn AJ, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;340:c363.
Lynch NE, Stevenson NJ, Livingstone V, et al. The temporal characteristics of seizures in neonatal hypoxic ischemic encephalopathy treated with hypothermia. Seizure. 2015;33:60–5.
Low E, Boylan GB, Mathieson SR, et al. Cooling and seizure burden in term neonates: an observational study. Arch Dis Child Fetal Neonatal Ed. 2012;97:F267–272.
Srinivasakumar P, Zempel J, Wallendorf M, Lawrence R, Inder T, Mathur A. Therapeutic hypothermia in neonatal hypoxic ischemic encephalopathy: electrographic seizures and magnetic resonance imaging evidence of injury. J Pediatr. 2013;163:465–70.
Harbert MJ, Tam EW, Glass HC, et al. Hypothermia is correlated with seizure absence in perinatal stroke. J Child Neurol. 2011;26:1126–30.
Mruk AL, Garlitz KL, Leung NR. Levetiracetam in neonatal seizures: a review. J Pediatr Pharmacol Ther. 2015;20:76–89.
Khan O, Chang E, Cipriani C, Wright C, Crisp E, Kirmani B. Use of intravenous levetiracetam for management of acute seizures in neonates. Pediatr Neurol. 2011;44:265–9.
Yau ML, Fung EL, Ng PC. Response of levetiracetam in neonatal seizures. World J Clin Pediatr. 2015;4:45–9.
Abend NS, Gutierrez-Colina AM, Monk HM, Dlugos DJ, Clancy RR. Levetiracetam for treatment of neonatal seizures. J Child Neurol. 2011;26:465–70.
Ramantani G, Ikonomidou C, Walter B, Rating D, Dinger J. Levetiracetam: safety and efficacy in neonatal seizures. Eur J Paediatr Neurol. 2011;15:1–7.
Maitre NL, Smolinsky C, Slaughter JC, Stark AR. Adverse neurodevelopmental outcomes after exposure to phenobarbital and levetiracetam for the treatment of neonatal seizures. J Perinatol. 2013;33:841–6.
Glass HC, Poulin C, Shevell MI. Topiramate for the treatment of neonatal seizures. Pediatr Neurol. 2011;44:439–42.
Weeke LC, Toet MC, van Rooij LG, et al. Lidocaine response rate in aEEG-confirmed neonatal seizures: retrospective study of 413 full-term and preterm infants. Epilepsia. 2016;57:233–42.
Weeke LC, Schalkwijk S, Toet MC, van Rooij LG, de Vries LS, van den Broek MP. Lidocaine-associated cardiac events in newborns with seizures: incidence, symptoms and contributing factors. Neonatology. 2015;108:130–6.
Kirmse K, Kummer M, Kovalchuk Y, Witte OW, Garaschuk O, Holthoff K. GABA depolarizes immature neurons and inhibits network activity in the neonatal neocortex in vivo. Nat Commun. 2015;6:7750.
Pressler RM, Boylan GB, Marlow N, et al. Bumetanide for the treatment of seizures in newborn babies with hypoxic ischaemic encephalopathy (NEMO): an open-label, dose finding, and feasibility phase 1/2 trial. Lancet Neurol. 2015;14:469–77. This study examined bumetanide as a novel therapeutic approach for seizures targeted to neonatal physiology, but reported negative results.
Thoresen M, Sabir H. Epilepsy: neonatal seizures still lack safe and effective treatment. Nat Rev Neurol. 2015;11:311–2.
Pressler RM, Boylan GB, Marlow N, et al. Bumetanide for neonatal seizures-back from the cotside. Nat Rev Neurol. 2015;11:724.
Glass HC. Bumetanide for treatment of seizures in neonates. Lancet Neurol. 2015;14:456–7.
Jamuar SS, Lam AT, Kircher M, et al. Somatic mutations in cerebral cortical malformations. N Engl J Med. 2014;371:733–43.
D’Gama AM, Geng Y, Couto JA, et al. Mammalian target of rapamycin pathway mutations cause hemimegalencephaly and focal cortical dysplasia. Ann Neurol. 2015;77:720–5.
Mirzaa GM, Poduri A. Megalencephaly and hemimegalencephaly: breakthroughs in molecular etiology. Am J Med Genet C: Semin Med Genet. 2014;166C:156–72.
Barcia G, Fleming MR, Deligniere A, et al. De novo gain-of-function KCNT1 channel mutations cause malignant migrating partial seizures of infancy. Nat Genet. 2012;44:1255–9.
Kim GE, Kronengold J, Barcia G, et al. Human slack potassium channel mutations increase positive cooperativity between individual channels. Cell Rep. 2014;9:1661–72.
Milligan CJ, Li M, Gazina EV, et al. KCNT1 gain of function in 2 epilepsy phenotypes is reversed by quinidine. Ann Neurol. 2014;75:581–90.
Bearden D, Strong A, Ehnot J, DiGiovine M, Dlugos D, Goldberg EM. Targeted treatment of migrating partial seizures of infancy with quinidine. Ann Neurol. 2014;76:457–61. This study examined is proof of principle that in vitro functional characterization of genetic mutation associated with epilepsy can lead to precision therapy.
Grinton BE, Heron SE, Pelekanos JT, et al. Familial neonatal seizures in 36 families: clinical and genetic features correlate with outcome. Epilepsia. 2015;56:1071–80.
Zara F, Specchio N, Striano P, et al. Genetic testing in benign familial epilepsies of the first year of life: clinical and diagnostic significance. Epilepsia. 2013;54:425–36.
Weckhuysen S, Ivanovic V, Hendrickx R, et al. Extending the KCNQ2 encephalopathy spectrum: clinical and neuroimaging findings in 17 patients. Neurology. 2013;81:1697–703.
Saitsu H, Kato M, Koide A, et al. Whole exome sequencing identifies KCNQ2 mutations in Ohtahara syndrome. Ann Neurol. 2012;72:298–300.
Milh M, Boutry-Kryza N, Sutera-Sardo J, et al. Similar early characteristics but variable neurological outcome of patients with a de novo mutation of KCNQ2. Orphanet J Rare Dis. 2013;8:80.
Kato M, Yamagata T, Kubota M, et al. Clinical spectrum of early onset epileptic encephalopathies caused by KCNQ2 mutation. Epilepsia. 2013;54:1282–7.
Weckhuysen S, Mandelstam S, Suls A, et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 2012;71:15–25.
Numis AL, Angriman M, Sullivan JE, et al. KCNQ2 encephalopathy: delineation of the electroclinical phenotype and treatment response. Neurology. 2014;82:368–70.
Schroeder BC, Kubisch C, Stein V, Jentsch TJ. Moderate loss of function of cyclic-AMP-modulated KCNQ2/KCNQ3 K+ channels causes epilepsy. Nature. 1998;396:687–90.
Miceli F, Soldovieri MV, Ambrosino P, et al. Genotype-phenotype correlations in neonatal epilepsies caused by mutations in the voltage sensor of K(v)7.2 potassium channel subunits. Proc Natl Acad Sci U S A. 2013;110:4386–91.
Orhan G, Bock M, Schepers D, et al. Dominant-negative effects of KCNQ2 mutations are associated with epileptic encephalopathy. Ann Neurol. 2014;75:382–94.
Miceli F, Soldovieri MV, Ambrosino P, et al. Early-onset epileptic encephalopathy caused by gain-of-function mutations in the voltage sensor of Kv7.2 and Kv7.3 potassium channel subunits. J Neurosci. 2015;35:3782–93.
Dedek K, Fusco L, Teloy N, Steinlein OK. Neonatal convulsions and epileptic encephalopathy in an Italian family with a missense mutation in the fifth transmembrane region of KCNQ2. Epilepsy Res. 2003;54:21–7.
Borgatti R, Zucca C, Cavallini A, et al. A novel mutation in KCNQ2 associated with BFNC, drug resistant epilepsy, and mental retardation. Neurology. 2004;63:57–65.
Steinlein OK, Conrad C, Weidner B. Benign familial neonatal convulsions: always benign? Epilepsy Res. 2007;73:245–9.
Miceli F, Striano P, Soldovieri MV, et al. A novel KCNQ3 mutation in familial epilepsy with focal seizures and intellectual disability. Epilepsia. 2015;56:e15–20.
Pisano T, Numis AL, Heavin SB, et al. Early and effective treatment of KCNQ2 encephalopathy. Epilepsia. 2015;56:685–91.
Howell KB, McMahon JM, Carvill GL, et al. SCN2A encephalopathy: a major cause of epilepsy of infancy with migrating focal seizures. Neurology. 2015;85:958–66.
Boerma RS, Braun KP, van de Broek MP, et al. Remarkable phenytoin sensitivity in 4 children with SCN8A-related epilepsy: a molecular neuropharmacological approach. Neurotherapeutics. 2016;13:192–7.
Larsen J, Carvill GL, Gardella E, et al. The phenotypic spectrum of SCN8A encephalopathy. Neurology. 2015;84:480–9.
Ohba C, Kato M, Takahashi S, et al. Early onset epileptic encephalopathy caused by de novo SCN8A mutations. Epilepsia. 2014;55:994–1000.
Nakamura K, Kato M, Osaka H, et al. Clinical spectrum of SCN2A mutations expanding to Ohtahara syndrome. Neurology. 2013;81:992–8.
Matalon D, Goldberg E, Medne L, Marsh ED. Confirming an expanded spectrum of SCN2A mutations: a case series. Epileptic Disord. 2014;16:13–8.
Stamberger H, Nikanorova M, Willemsen MH, et al. STXBP1 encephalopathy: a neurodevelopmental disorder including epilepsy. Neurology. 2016;86:954–62.
Rehman A, Archbold JK, Hu SH, Norwood SJ, Collins BM, Martin JL. Reconciling the regulatory role of Munc18 proteins in SNARE-complex assembly. IUCrJ. 2014;1:505–13.
Atwal PS, Scaglia F. Molybdenum cofactor deficiency. Mol Genet Metab. 2016;117:1–4.
Schwahn BC, Van Spronsen FJ, Belaidi AA, et al. Efficacy and safety of cyclic pyranopterin monophosphate substitution in severe molybdenum cofactor deficiency type A: a prospective cohort study. Lancet. 2015;386:1955–63.
van Karnebeek CD, Tiebout SA, Niermeijer J, et al. Pyridoxine-dependent epilepsy: an expanding clinical spectrum. Pediatr Neurol. 2016;59:6–12.
van Karnebeek CD, Jaggumantri S. Current treatment and management of pyridoxine-dependent epilepsy. Curr Treat Options Neurol. 2015;17:335.
Gallagher RC, Van Hove JL, Scharer G, et al. Folinic acid-responsive seizures are identical to pyridoxine-dependent epilepsy. Ann Neurol. 2009;65:550–6.
Cirillo M, Venkatesan C, Millichap JJ, Stack CV, Nordli Jr DR. Case report: intravenous and oral pyridoxine trial for diagnosis of pyridoxine-dependent epilepsy. Pediatrics. 2015;136:e257–261.
Bok LA, Maurits NM, Willemsen MA, et al. The EEG response to pyridoxine-IV neither identifies nor excludes pyridoxine-dependent epilepsy. Epilepsia. 2010;51:2406–11.
Bok LA, Halbertsma FJ, Houterman S, et al. Long-term outcome in pyridoxine-dependent epilepsy. Dev Med Child Neurol. 2012;54:849–54.
Basura GJ, Hagland SP, Wiltse AM, Gospe Jr SM. Clinical features and the management of pyridoxine-dependent and pyridoxine-responsive seizures: review of 63 North American cases submitted to a patient registry. Eur J Pediatr. 2009;168:697–704.
Coughlin 2nd CR, van Karnebeek CD, Al-Hertani W, et al. Triple therapy with pyridoxine, arginine supplementation and dietary lysine restriction in pyridoxine-dependent epilepsy: neurodevelopmental outcome. Mol Genet Metab. 2015;116:35–43.
Mills PB, Surtees RA, Champion MP, et al. Neonatal epileptic encephalopathy caused by mutations in the PNPO gene encoding pyridox(am)ine 5’-phosphate oxidase. Hum Mol Genet. 2005;14:1077–86.
Hoffmann GF, Schmitt B, Windfuhr M, et al. Pyridoxal 5’-phosphate may be curative in early-onset epileptic encephalopathy. J Inherit Metab Dis. 2007;30:96–9.
Pisani F, Spagnoli C. Neonatal seizures: a review of outcomes and outcome predictors. Neuropediatrics. 2016;47:12–9.
Soltirovska-Salamon A, Neubauer D, Petrovcic A, Paro-Panjan D. Risk factors and scoring system as a prognostic tool for epilepsy after neonatal seizures. Pediatr Neurol. 2014;50:77–84.
Galanopoulou AS, Moshe SL. In search of epilepsy biomarkers in the immature brain: goals, challenges and strategies. Biomark Med. 2011;5:615–28.
Simonato M, Brooks-Kayal AR, Engel Jr J, et al. The challenge and promise of anti-epileptic therapy development in animal models. Lancet Neurol. 2014;13:949–60.
Fox CK, Glass HC, Sidney S, Smith SE, Fullerton HJ. Neonatal seizures triple the risk of a remote seizure after perinatal ischemic stroke. Neurology. 2016;86:2179–86. This article highlights the risk of post-neonatal epilepsy, up to the first decade, specifically in neonates with perinatal ischemic stroke, the second most common cause of neonatal seizures.
Suppiej A, Mastrangelo M, Mastella L, et al. Pediatric epilepsy following neonatal seizures symptomatic of stroke. Brain Dev. 2016;38:27–31.
Clancy RR, Legido A. Postnatal epilepsy after EEG-confirmed neonatal seizures. Epilepsia. 1991;32:69–76.
Pisani F, Piccolo B, Cantalupo G, et al. Neonatal seizures and postneonatal epilepsy: a 7-y follow-up study. Pediatr Res. 2012;72:186–93.
Venkatesan C, Millichap JJ, Krueger JM, et al. Epilepsy following neonatal seizures secondary to hemorrhagic stroke in term neonates. J Child Neurol. 2016;31:547–52.
Martinez-Biarge M, Diez-Sebastian J, Kapellou O, et al. Predicting motor outcome and death in term hypoxic-ischemic encephalopathy. Neurology. 2011;76:2055–61.
da Jung E, Ritacco DG, Nordli DR, Koh S, Venkatesan C. Early anatomical injury patterns predict epilepsy in head cooled neonates with hypoxic-ischemic encephalopathy. Pediatr Neurol. 2015;53:135–40.
Twomey E, Twomey A, Ryan S, Murphy J, Donoghue VB. MR imaging of term infants with hypoxic-ischaemic encephalopathy as a predictor of neurodevelopmental outcome and late MRI appearances. Pediatr Radiol. 2010;40:1526–35.
Jose A, Matthai J, Paul S. Correlation of EEG, CT, and MRI brain with neurological outcome at 12 months in term newborns with hypoxic ischemic encephalopathy. J Clin Neonatol. 2013;2:125–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Tristan T. Sands and Tiffani L. McDonough declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Epilepsy
Rights and permissions
About this article
Cite this article
Sands, T.T., McDonough, T.L. Recent Advances in Neonatal Seizures. Curr Neurol Neurosci Rep 16, 92 (2016). https://doi.org/10.1007/s11910-016-0694-x
Published:
DOI: https://doi.org/10.1007/s11910-016-0694-x