Abstract
Purpose of Review
Despite the availability of various medications and prescribing combination therapies, uncontrolled blood pressure and resistance are observed in more than 40% of patients. The purpose of this review is to discuss emerging novel approaches for the treatment of hypertension and propose future research and clinical directions.
Recent Findings
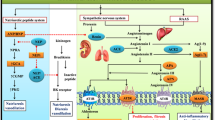
Hypertension is a common disease of the cardiovascular system which may arise solely or as a comorbidity of other disorders. It is a crucial risk factor for cardiovascular diseases such as coronary artery disease, myocardial infarction, congestive heart failure, renal failure, and stroke. The results from current literature regarding the novel approaches showed several targets that could be explored as potential therapeutic options. These include toll-like receptor 4, a critical regulator of angiotensin II–induced hypertension; protease-activated receptor 2, which promotes collagen deposition and inflammatory responses; chemerin, which causes metabolic and obesity-associated hypertension; apelin receptor; transient receptor potential melastatin; urotensin-II; and Tie2 receptor.
Summary
This review discusses various targets and pathways that could be emerging pharmacological therapies for hypertension.







Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension. Eur Heart J. 2013;34:2159–219 Available from: https://academic.oup.com/eurheartj/article-lookup/doi/10.1093/eurheartj/eht151.
•• Chow CK, Gupta R. Blood pressure control: a challenge to global health systems [Internet]. Lancet. Lancet Publishing Group; 2019 [cited 2021 Feb 1]. p. 613–5. Available from: https://doi.org/10.1016/S0140-6736(19)31293-0. This paper is a commentary on increasing global burden of hypertension and challenges associated with this.
•• Tocci G, Presta V, Ferri C, Redon J, Volpe M. Blood pressure targets achievement according to 2018 ESC/ESH guidelines in three European excellence centers for hypertension. High Blood Press Cardiovasc Prev [Internet]. Springer International Publishing; 2020;27:51–9. Available from: https://doi.org/10.1007/s40292-020-00359-0. This is an important paper showing that proportions of treated uncontrolled hypertensive patients is substantially increased.
Tocci G, Presta V, Citoni B, Figliuzzi I, Bianchi F, Ferrucci A, et al. Blood pressure target achievement under monotherapy: a real-life appraisal. High Blood Press Cardiovasc Prev. 2020;27:587–96. Springer International Publishing Available from. https://doi.org/10.1007/s40292-020-00420-y.
Sim JJ, Bhandari SK, Shi J, Liu ILA, Calhoun DA, McGlynn EA, et al. Characteristics of resistant hypertension in a large, ethnically diverse hypertension population of an integrated health system. Mayo Clin Proc. 2013;88:1099–107 Elsevier Inc. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0025619613005636.
Carey RM, Sakhuja S, Calhoun DA, Whelton PK, Muntner P. Prevalence of apparent treatment-resistant hypertension in the United States. Hypertension. 2019;73:424–31 Available from: https://www.ahajournals.org/doi/10.1161/HYPERTENSIONAHA.118.12191.
Ceral J, Habrdova V, Vorisek V, Bima M, Pelouch R, Solar M. Difficult-to-control arterial hypertension or uncooperative patients? The assessment of serum antihypertensive drug levels to differentiate non-responsiveness from non-adherence to recommended therapy. Hypertens Res. 2011;34:87–90 Nature Publishing Group. Available from: http://www.nature.com/articles/hr2010183.
Jung O, Gechter JL, Wunder C, Paulke A, Bartel C, Geiger H, et al. Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens. 2013;31:766–74 Available from: http://journals.lww.com/00004872-201304000-00019.
Oparil S, Schmieder RE. New approaches in the treatment of hypertension. Circ. Res. Lippincott Williams and Wilkins; 2015 [cited 2021 Mar 4]. p. 1074–95. Available from: http://circres.ahajournals.org
O’Neill LA. How Toll-like receptors signal: what we know and what we don’t know. Curr Opin Immunol. 2006;18:3–9 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0952791505002050.
Faure E, Thomas L, Xu H, Medvedev AE, Equils O, Arditi M. Bacterial lipopolysaccharide and IFN-γ induce Toll-like receptor 2 and Toll-like receptor 4 expression in human endothelial cells: role of NF-κB activation. J Immunol. 2001;166:2018–24 Available from: http://www.jimmunol.org/lookup/doi/10.4049/jimmunol.166.3.2018.
Yang X, Coriolan D, Murthy V, Schultz K, Golenbock DT, Beasley D. Proinflammatory phenotype of vascular smooth muscle cells: role of efficient Toll-like receptor 4 signaling. Am J Physiol Circ Physiol. 2005;289:H1069–76 Available from: https://www.physiology.org/doi/10.1152/ajpheart.00143.2005.
Zeuke S. TLR4-mediated inflammatory activation of human coronary artery endothelial cells by LPS. Cardiovasc Res. 2002;56:126–34 Available from: https://academic.oup.com/cardiovascres/article-lookup/doi/10.1016/S0008-6363(02)00512-6.
Eißler R, Schmaderer C, Rusai K, Kühne L, Sollinger D, Lahmer T, et al. Hypertension augments cardiac Toll-like receptor 4 expression and activity. Hypertens Res. 2011;34:551–8 Available from: http://www.nature.com/articles/hr2010270.
Pushpakumar S, Ren L, Kundu S, Gamon A, Tyagi SC, Sen U. Toll-like receptor 4 deficiency reduces oxidative stress and macrophage mediated inflammation in hypertensive kidney. Sci Rep. 2017;7:6349 Springer US Available from: http://www.nature.com/articles/s41598-017-06484-6.
Bomfim GF, Dos Santos RA, Oliveira MA, Giachini FR, Akamine EH, Tostes RC, et al. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin Sci. 2012;122:535–43 Available from: https://portlandpress.com/clinsci/article/122/11/535/68943/Tolllike-receptor-4-contributes-to-blood-pressure.
• Hernanz R, Martínez-Revelles S, Palacios R, Martín A, Cachofeiro V, Aguado A, et al. Toll-like receptor 4 contributes to vascular remodelling and endothelial dysfunction in angiotensin II-induced hypertension. Br J Pharmacol. 2015;172:3159–76 Available from: http://doi.wiley.com/10.1111/bph.13117. This paper provides evidences for the role of TLR4 in endothelial dysfunction in Ang II–induced hypertension.
Chen X, Shi X, Zhang X, Lei H, Long S, Su H, et al. Scutellarin Attenuates Hypertension-induced expression of brain Toll-like receptor 4/nuclear factor kappa B. Mediat Inflamm. 2013;2013:1–9 Available from: https://www.hindawi.com/journals/mi/2013/432623/.
Ichikawa H, Shimada M, Narita M, Narita I, Kimura Y, Tanaka M, et al. Rivaroxaban, a direct factor Xa inhibitor, ameliorates hypertensive renal damage through inhibition of the inflammatory response mediated by protease-activated receptor pathway. J Am Heart Assoc. 2019;8:1–14 Available from: https://www.ahajournals.org/doi/10.1161/JAHA.119.012195.
McLarty JL, Meléndez GC, Brower GL, Janicki JS, Levick SP. Tryptase/protease-activated receptor 2 interactions induce selective mitogen-activated protein kinase signaling and collagen synthesis by cardiac fibroblasts. Hypertension. 2011;58:264–70 Available from: https://www.ahajournals.org/doi/10.1161/HYPERTENSIONAHA.111.169417.
Steinberg SF. The cardiovascular actions of protease-activated rceptors. Mol Pharmacol: Am Soc Pharmacol Exp Ther. 2005;67:2–11 Available from: http://molpharm.aspetjournals.org/lookup/doi/10.1124/mol.104.003103.
Lindner JR, Kahn ML, Coughlin SR, Sambrano GR, Schauble E, Bernstein D, et al. Delayed Onset of inflammation in protease-activated receptor-2-deficient mice. J Immunol: Am Assoc Immunol. 2000;165:6504–10 Available from: http://www.jimmunol.org/lookup/doi/10.4049/jimmunol.165.11.6504.
Vergnolle N, Bunnett NW, Sharkey KA, Brussee V, Compton SJ, Grady EF, et al. Proteinase-activated receptor-2 and hyperalgesia: a novel pain pathway. Nat Med. 2001;7:821–6 Available from: http://www.nature.com/articles/nm0701_821.
• Ando M, Matsumoto T, Kobayashi S, Iguchi M, Taguchi K, Kobayashi T. Impairment of protease-activated receptor 2-induced relaxation of aortas of aged spontaneously hypertensive rat. Biol Pharm Bull. 2018;41:815–9 Available from: https://www.jstage.jst.go.jp/article/bpb/41/5/41_b17-00987/_article. Results of this study suggested role of PAR-2 in hypertension.
He R-Q, Tang X-F, Zhang B-L, Li X-D, Hong M-N, Chen Q-Z, et al. Protease-activated receptor 1 and 2 contribute to angiotensin II-induced activation of adventitial fibroblasts from rat aorta. Biochem Biophys Res Commun. 2016;473:517–23 Elsevier Ltd. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0006291X16304053.
Xu L, Brink M. mTOR, cardiomyocytes and inflammation in cardiac hypertrophy. Biochim Biophys Acta, Mol Cell Res. 2016;1863:1894–903 Elsevier B.V. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0167488916000069.
Saxton RA, Sabatini DM. mTOR Signaling in growth, metabolism, and disease. Cell. 2017;168:960–76 Elsevier Inc. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0092867417301824.
Gleason CE, Frindt G, Cheng C-J, Ng M, Kidwai A, Rashmi P, et al. mTORC2 regulates renal tubule sodium uptake by promoting ENaC activity. J Clin Invest. 2015;125:117–28 Available from: http://www.jci.org/articles/view/73935.
Brown EJ, Albers MW, Bum Shin T, Ichikawa K, Keith CT, Lane WS, et al. A mammalian protein targeted by G1-arresting rapamycin–receptor complex. Nature. 1994;369:756–8 Available from: http://www.nature.com/articles/369756a0.
Sabers CJ, Martin MM, Brunn GJ, Williams JM, Dumont FJ, Wiederrecht G, et al. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J Biol Chem. 1995;270:815–22 Available from: http://www.jbc.org/lookup/doi/10.1074/jbc.270.2.815.
Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0092867406001085.
Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW-L, Thomas EL, et al. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–65 Elsevier Ltd; Available from: https://linkinghub.elsevier.com/retrieve/pii/S1550413110001531.
Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93 Elsevier; Available from: https://linkinghub.elsevier.com/retrieve/pii/S0092867412003510.
Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci. 2013;126:1713–9 Available from: http://jcs.biologists.org/cgi/doi/10.1242/jcs.125773.
Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–45 Available from: http://www.nature.com/articles/nature11861.
Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: A mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43 Available from: https://linkinghub.elsevier.com/retrieve/pii/0092867494905703.
Kumar V, Wollner C, Kurth T, Bukowy JD, Cowley AW. Inhibition of mammalian target of rapamycin complex 1 attenuates salt-induced hypertension and kidney injury in Dahl salt-sensitive rats. Hypertension. 2017;70:813–21 Available from: https://www.ahajournals.org/doi/10.1161/HYPERTENSIONAHA.117.09456.
Wang W, Wang H, Geng Q-X, Wang H-T, Miao W, Cheng B, et al. Augmentation of autophagy by atorvastatin via Akt/mTOR pathway in spontaneously hypertensive rats. Hypertens Res [Internet]. Nat Publ Group. 2015;38:813–20 Available from: http://www.nature.com/articles/hr201585.
Li Y, Yang L, Dong L, Yang Z, Zhang J, Zhang S, et al. Crosstalk between the Akt/mTORC1 and NF-κB signaling pathways promotes hypoxia-induced pulmonary hypertension by increasing DPP4 expression in PASMCs. Acta Pharmacol Sin. 2019;40:1322–33 Springer US; Available from: http://www.nature.com/articles/s41401-019-0272-2.
• Kumar V, Evans LC, Kurth T, Yang C, Wollner C, Nasci V, et al. Therapeutic suppression of mTOR (mammalian target of rapamycin) signaling prevents and reverses salt-induced hypertension and kidney injury in Dahl salt-sensitive rats. Hypertension. 2019;73:630–9 Available from: https://www.ahajournals.org/doi/10.1161/HYPERTENSIONAHA.118.12378. The data from this paper reveals the role of mTOR and its pathway components for sodium balance and arterial BP regulation.
Bozaoglu K, Curran JE, Stocker CJ, Zaibi MS, Segal D, Konstantopoulos N, et al. Chemerin, a novel adipokine in the regulation of angiogenesis. J Clin Endocrinol Metab. 2010;95:2476–85 Endocrine Society; Available from: https://academic.oup.com/jcem/article/95/5/2476/2597606.
Chu SH, Lee MK, Ahn KY, Im J-A, Park MS, Lee D-C, et al. Chemerin and adiponectin contribute reciprocally to metabolic syndrome. Ryffel B, editor. PLoS One [Internet]. 2012;7:e34710. Available from: https://dx.plos.org/10.1371/journal.pone.0034710
Mengliu Y, Gangyi Y, Jing D, Ying L, Haihong Z, Hua L, et al. Elevated plasma levels of chemerin in newly diagnosed type 2 diabetes mellitus with hypertension. J Investig Med. BMJ Publishing Group. 2010;58:883–6.
Watts SW, Dorrance AM, Penfold ME, Rourke JL, Sinal CJ, Seitz B, et al. Chemerin connects fat to arterial contraction. Arterioscler Thromb Vasc Biol. 2013;33:1320–8 Available from: https://www.ahajournals.org/doi/10.1161/ATVBAHA.113.301476.
Kunimoto H, Kazama K, Takai M, Oda M, Okada M, Yamawaki H. Chemerin promotes the proliferation and migration of vascular smooth muscle and increases mouse blood pressure. Am J Physiol Circ Physiol. 2015;309:H1017–28 Available from: https://www.physiology.org/doi/10.1152/ajpheart.00820.2014.
Wen J, Wang J, Guo L, Cai W, Wu Y, Chen W, et al. Chemerin stimulates aortic smooth muscle cell proliferation and migration via activation of autophagy in VSMCs of metabolic hypertension rats. Am J Transl Res. 2019;11:1327–42 Available from: http://www.ncbi.nlm.nih.gov/pubmed/30972165.
Weng C, Shen Z, Li X, Jiang W, Peng L, Yuan H, et al. Effects of chemerin/CMKLR1 in obesity-induced hypertension and potential mechanism. Am J Transl Res. 2017;9:3096–104 Available from: http://www.ncbi.nlm.nih.gov/pubmed/28670396.
• Kennedy AJ, Yang P, Read C, Kuc RE, Yang L, Taylor EJA, et al. Chemerin elicits potent constrictor actions via chemokine-like receptor 1 (CMKLR1), not G-protein-coupled receptor 1 (GPR1), in human and rat vasculature. J Am Heart Assoc. 2016;5:1–15 Available from: https://www.ahajournals.org/doi/10.1161/JAHA.116.004421. This paper provides important finding that C9 chemerin increases contraction and pulmonary vascular resistance in arteries with increased BP.
Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou M-X, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–6 Academic Press Inc.; Available from: https://linkinghub.elsevier.com/retrieve/pii/S0006291X9899489X.
Chandra SM, Razavi H, Kim J, Agrawal R, Kundu RK, de Jesus PV, et al. Disruption of the apelin-APJ system worsens hypoxia-induced pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2011;31:814–20 Available from: https://www.ahajournals.org/doi/10.1161/ATVBAHA.110.219980.
Goetze JP, Rehfeld JF, Carlsen J, Videbaek R, Andersen CB, Boesgaard S, et al. Apelin: A new plasma marker of cardiopulmonary disease. Regul Pept. 2006;133:134–8 Available from: https://linkinghub.elsevier.com/retrieve/pii/S016701150500234X.
Griffiths PR, Lolait SJ, Harris LE, Paton JFR, O’Carroll A-M. Vasopressin V1a receptors mediate the hypertensive effects of [Pyr 1 ]apelin-13 in the rat rostral ventrolateral medulla. J Physiol. 2017;595:3303–18 Available from: http://doi.wiley.com/10.1113/JP274178.
O’Carroll A-M, Lolait SJ, Harris LE, Pope GR. The apelin receptor APJ: journey from an orphan to a multifaceted regulator of homeostasis. J Endocrinol. 2013;219:R13–35 Available from: https://joe.bioscientifica.com/view/journals/joe/219/1/R13.xml.
Reaux A, Gallatz K, Palkovits M, Llorens-Cortes C. Distribution of apelin-synthesizing neurons in the adult rat brain. Neuroscience. 2002;113:653–62 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0306452202001926.
O’Carroll A-M, Lolait SJ. Regulation of rat APJ receptor messenger ribonucleic acid expression in magnocellular neurones of the paraventricular and supraopric nuclei by osmotic stimuli. J Neuroendocrinol. 2003;15:661–6 Available from: http://doi.wiley.com/10.1046/j.1365-2826.2003.01044.x.
Yang P, Maguire JJ, Davenport AP. Apelin, Elabela/Toddler, and biased agonists as novel therapeutic agents in the cardiovascular system. Trends Pharmacol Sci. 2015;36:560–7 Elsevier Ltd; Available from: https://linkinghub.elsevier.com/retrieve/pii/S0165614715001170.
• Yang P, Read C, Kuc RE, Nyimanu D, Williams TL, Crosby A, et al. A novel cyclic biased agonist of the apelin receptor, MM07, is disease modifying in the rat monocrotaline model of pulmonary arterial hypertension. Br J Pharmacol. 2019;176:1206–21 Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/bph.14603This paper suggest the role of apelin receptor on pulmonary hypertension and proposed that G protein-based agonism of the apelin receptor has a beneficial effect in PAH.
Wang C, Liu X, Kong D, Qin X, Li Y, Teng X, et al. Apelin as a novel drug for treating preeclampsia. Exp Ther Med. 2017:5917–23 Available from: http://www.spandidos-publications.com/10.3892/etm.2017.5304.
Simard JM, Woo SK, Gerzanich V. Transient receptor potential melastatin 4 and cell death. Pflugers Arch - Eur J Physiol. 2012;464:573–82 Available from: http://link.springer.com/10.1007/s00424-012-1166-z.
Abriel H, Syam N, Sottas V, Amarouch MY, Rougier J-S. TRPM4 channels in the cardiovascular system: physiology, pathophysiology, and pharmacology. Biochem Pharmacol. 2012;84:873–81 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0006295212004194.
Becerra A, Echeverría C, Varela D, Sarmiento D, Armisén R, Nuñez-Villena F, et al. Transient receptor potential melastatin 4 inhibition prevents lipopolysaccharide-induced endothelial cell death. Cardiovasc Res. 2011;91:677–84 Available from: https://academic.oup.com/cardiovascres/article-lookup/doi/10.1093/cvr/cvr135.
• Ding X-Q, Ban T, Liu Z-Y, Lou J, Tang L-L, Wang J-X, et al. Transient receptor potential melastatin 4 (TRPM4) contributes to high salt diet-mediated early-stage endothelial injury. Cell Physiol Biochem. 2017;41:835–48 Available from: https://www.karger.com/Article/FullText/459695This paper provide evidences that TRPM4 is associated with high salt diet-mediated early-stage endothelial injury.
McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–8 Available from: http://www.nature.com/articles/nature719.
Calixto J, Kassuya C, Andre E, Ferreira J. Contribution of natural products to the discovery of the transient receptor potential (TRP) channels family and their functions. Pharmacol Ther. 2005;106:179–208 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0163725804002049.
Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–15 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0092867402006529.
Mathar I, Vennekens R, Meissner M, Kees F, Van der Mieren G, Camacho Londoño JE, et al. Increased catecholamine secretion contributes to hypertension in TRPM4-deficient mice. J Clin Invest. 2010;120:3267–79 Available from: http://www.jci.org/articles/view/41348.
Huang F, Ni M, Zhang J-M, Li D-J, Shen F-M. TRPM8 downregulation by angiotensin II in vascular smooth muscle cells is involved in hypertension. Mol Med Rep. 2017;15:1900–8 Available from: https://www.spandidos-publications.com/10.3892/mmr.2017.6158.
• Xiong S, Wang B, Lin S, Zhang H, Li Y, Wei X, et al. Activation of transient receptor potential melastatin subtype 8 attenuates cold-induced hypertension through ameliorating vascular mitochondrial dysfunction. J Am Heart Assoc. 2017;6:1–18 Available from: https://www.ahajournals.org/doi/10.1161/JAHA.117.005495The results of this study suggested that TRPM 8 activation attenuated cold-induced hypertension in rats.
Effects and safety of menthol on blood pressure and metabolic parameters in prehypertensive and mild hypertensive patients - full text view - ClinicalTrials.gov [Internet]. [cited 2021 Feb 1]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT01408446?term=trpm&cond=Hypertension&draw=2&rank=1
Maguire JJ, Davenport AP. Is urotensin-II the new endothelin? Br J Pharmacol. 2002;137:579–88 Wiley-Blackwell. Available from: http://doi.wiley.com/10.1038/sj.bjp.0704924.
Wu F, Chen G, Zhang A, Yu Y, Fan M, Tang C. Renal urotensin II system plays roles in the regulation of blood pressure in Dahl salt-resistant rat. Int J Hypertens. 2016;2016:1–11 Available from: https://www.hindawi.com/journals/ijhy/2016/9146870/.
Disa J, Floyd LE, Edwards RM, Douglas SA, Aiyar NV. Identification and characterization of binding sites for human urotensin-II in Sprague–Dawley rat renal medulla using quantitative receptor autoradiography. Peptides. 2006;27:1532–7 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0196978105004730.
• Nishi M, Yonesu K, Tagawa H, Kato M, Marumoto S, Nagayama T. A novel and highly potent urotensin II receptor antagonist inhibits urotensin II–induced pressure response in mice. J Cardiovasc Pharmacol. 2019;73:15–21 Available from: http://journals.lww.com/00005344-201901000-00003. This study provides evidences that urotensin II receptor can be target for hypertension therapy and urotensin II receptor antagonist inhibited urotensin II–induced pressure response.
Lee JH, Park BK, Oh K, Yi KY, Lim CJ, Seo HW, et al. A urotensin II receptor antagonist, KR36676, decreases vascular remodeling and inflammation in experimental pulmonary hypertension. Int Immunopharmacol. 2016;40:196–202 Elsevier B.V. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1567576916303770.
Chan P, Hong H-J, Cheng T-H. 945 Tanshinone IIA reduces urotensin II signaling and compromised migration in cultured vascular smooth muscle cells. J Hypertens. 2012;30:e273 Available from: http://journals.lww.com/00004872-201209001-00871.
Yao S, Su C, Wu S-H, Hu D-J, Liu X. Aliskiren improved the endothelial repair capacity of endothelial progenitor cells from patients with hypertension via the Tie2/PI3k/Akt/eNOS signalling pathway. Cardiol Res Pract. 2020;2020:1–11 Available from: https://www.hindawi.com/journals/crp/2020/6534512/.
Lee J-S, Song S-H, Kim J-M, Shin I-S, Kim KL, Suh Y-L, et al. Angiopoietin-1 prevents hypertension and target organ damage through its interaction with endothelial Tie2 receptor. Cardiovasc Res. 2008;78:572–80 Available from: https://academic.oup.com/cardiovascres/article-lookup/doi/10.1093/cvr/cvn048.
Yamamoto A, Takahashi H, Kojima Y, Tsuda Y, Morio Y, Muramatsu M, et al. Downregulation of angiopoietin-1 and Tie2 in chronic hypoxic pulmonary hypertension. Respiration. 2008;75:328–38 Available from: https://www.karger.com/Article/FullText/112432.
Kugathasan L, Ray JB, Deng Y, Rezaei E, Dumont DJ, Stewart DJ. The angiopietin-1–Tie2 pathway prevents rather than promotes pulmonary arterial hypertension in transgenic mice. J Exp Med. 2009;206:2221–34 Available from: https://rupress.org/jem/article/206/10/2221/46076/The-angiopietin1Tie2-pathway-prevents-rather-than.
Author information
Authors and Affiliations
Contributions
Conceptualization: Lokesh Kumar Bhatt and Tahir Hussain; Writing, original draft preparation: Ishant Selokar and Dezaree Raut; Writing, review and editing: Lokesh Kumar Bhatt and Tahir Hussain.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hypertension and Emergency Medicine
Rights and permissions
About this article
Cite this article
Bhatt, L.K., Selokar, I., Raut, D. et al. Novel Targets for Hypertension Drug Discovery. Curr Hypertens Rep 23, 19 (2021). https://doi.org/10.1007/s11906-021-01137-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s11906-021-01137-6