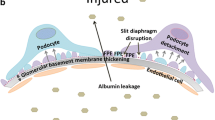
Abstract
Purpose of Review
The underlining goal of this review is to offer a concise, detailed look into current knowledge surrounding transient receptor potential canonical channel 6 (TRPC6) in the progression of diabetic kidney disease (DKD).
Recent Findings
Mutations and over-activation in TRPC6 channel activity lead to the development of glomeruli injury. Angiotensin II, reactive oxygen species, and other factors in the setting of DKD stimulate drastic increases in calcium influx through the TRPC6 channel, causing podocyte hypertrophy and foot process effacement. Loss of the podocytes further promote deterioration of the glomerular filtration barrier and play a major role in the development of both albuminuria and the renal injury in DKD. Recent genetic manipulation with TRPC6 channels in various rodent models provide additional knowledge about the role of TRPC6 in DKD and are reviewed here.
Summary
The TRPC6 channel has a pronounced role in the progression of DKD, with deviations in activity yielding detrimental outcomes. The benefits of targeting TRPC6 or its upstream or downstream signaling pathways in DKD are prominent.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Dryer SE, Roshanravan H, Kim EY. TRPC channels: Regulation, dysregulation and contributions to chronic kidney diseaseBiochim Biophys Acta Mol basis Dis. 2019. https://doi.org/10.1016/j.bbadis.2019.04.001 Excellent recent review summarizing the role of TRPC6 and other TRPC channels in regulation of renal function. Nice discussion of contradictory topics in the area.
Staruschenko A. Hypertension and diabetes mellitus: the chicken and egg problem. Hypertension. 2017;69(5):787–8. https://doi.org/10.1161/HYPERTENSIONAHA.117.08671.
DeMarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol. 2014;10(6):364–76. https://doi.org/10.1038/nrendo.2014.44.
Rieg T, Vallon V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia. 2018;61(10):2079–86. https://doi.org/10.1007/s00125-018-4654-7.
Vallon V, Thomson SC. Cardiovascular and renal benefits of SGLT2 inhibition: insights from CANVAS. Nat Rev Nephrol. 2017;13:517. https://doi.org/10.1038/nrneph.2017.113.
Giugliano D, De Nicola L, Maiorino MI, Bellastella G, Esposito K. Type 2 diabetes and the kidney: insights from cardiovascular outcome trials. Diabetes Obes Metab. 2019. https://doi.org/10.1111/dom.13743.
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019. https://doi.org/10.1056/NEJMoa1811744.
Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, Progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–45. https://doi.org/10.2215/cjn.11491116.
Chaudhari S, Ma R. Store-operated calcium entry and diabetic complications. Exp Biol Med. 2016;241(4):343–52. https://doi.org/10.1177/1535370215609693.
Wang Z, do Carmo JM, Aberdein N, Zhou X, Williams JM, da Silva AA, et al. Synergistic interaction of hypertension and diabetes in promoting kidney injury and the role of endoplasmic reticulum stress. Hypertension. 2017;69(5):879–91. https://doi.org/10.1161/HYPERTENSIONAHA.116.08560.
Trebak M, Putney JW Jr. ORAI calcium channels. Physiology. 2017;32(4):332–42. https://doi.org/10.1152/physiol.00011.2017.
Wang Y, Deng X, Gill DL. Calcium signaling by STIM and Orai: intimate coupling details revealed. Sci Signal. 2010;3(148):pe42. https://doi.org/10.1126/scisignal.3148pe42.
Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci U S A. 2007;104(11):4682–7. https://doi.org/10.1073/pnas.0611692104.
Liao Y, Plummer NW, George MD, Abramowitz J, Zhu MX, Birnbaumer L. A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. Proc Natl Acad Sci U S A. 2009;106(9):3202–6. https://doi.org/10.1073/pnas.0813346106.
Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009;23(2):297–328. https://doi.org/10.1096/fj.08-119495.
Ma R, Chaudhari S, Li W. Canonical transient receptor potential 6 channel: a new target of reactive oxygen species in renal physiology and pathology. Antioxid Redox Signal. 2016;25(13):732–48. https://doi.org/10.1089/ars.2016.6661.
Ilatovskaya DV, Staruschenko A. TRPC6 channel as an emerging determinant of the podocyte injury susceptibility in kidney diseases. Am J Physiol Renal Physiol. 2015;309(5):F393–7. https://doi.org/10.1152/ajprenal.00186.2015.
Dryer SE, Kim EY. Permeation and rectification in canonical transient receptor potential-6 (TRPC6) channels. Front Physiol. 2018;9:1055. https://doi.org/10.3389/fphys.2018.01055.
Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397(6716):259–63. https://doi.org/10.1038/16711.
Venkatachalam K, Zheng F, Gill DL. Regulation of canonical transient receptor potential (TRPC) channel function by diacylglycerol and protein kinase C. J Biol Chem. 2003;278(31):29031–40. https://doi.org/10.1074/jbc.M302751200.
Bousquet SM, Monet M, Boulay G. Protein kinase C-dependent phosphorylation of transient receptor potential canonical 6 (TRPC6) on serine 448 causes channel inhibition. J Biol Chem. 2010;285(52):40534–43. https://doi.org/10.1074/jbc.M110.160051.
Inoue R, Jensen LJ, Jian Z, Shi J, Hai L, Lurie AI, et al. Synergistic activation of vascular TRPC6 channel by receptor and mechanical stimulation via phospholipase C/diacylglycerol and phospholipase A2/omega-hydroxylase/20-HETE pathways. Circ Res. 2009;104(12):1399–409. https://doi.org/10.1161/circresaha.108.193227.
Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308(5729):1801–4.
Reiser J, Polu KR, Moller CC, Kenlan P, Altintas MM, Wei C, et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37(7):739–44.
Heeringa SF, Moller CC, Du J, Yue L, Hinkes B, Chernin G, et al. A novel TRPC6 mutation that causes childhood FSGS. PLoS One. 2009;4(11):e7771.
Buscher AK, Kranz B, Buscher R, Hildebrandt F, Dworniczak B, Pennekamp P, et al. Immunosuppression and renal outcome in congenital and pediatric steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2010;5(11):2075–84. https://doi.org/10.2215/cjn.01190210.
Riehle M, Buscher AK, Gohlke BO, Kassmann M, Kolatsi-Joannou M, Brasen JH, et al. TRPC6 G757D loss-of-function mutation associates with FSGS. J Am Soc Nephrol. 2016;27(9):2771–83. https://doi.org/10.1681/ASN.2015030318.
Obeidova L, Reiterova J, Lnenicka P, Stekrova J, Safrankova H, Kohoutova M, et al. TRPC6 gene variants in Czech adult patients with focal segmental glomerulosclerosis and minimal change disease. Folia Biol. 2012;58(4):173–6.
Barua M, Brown EJ, Charoonratana VT, Genovese G, Sun H, Pollak MR. Mutations in the INF2 gene account for a significant proportion of familial but not sporadic focal and segmental glomerulosclerosis. Kidney Int. 2013;83(2):316–22. https://doi.org/10.1038/ki.2012.349.
Santin S, Ars E, Rossetti S, Salido E, Silva I, Garcia-Maset R, et al. TRPC6 mutational analysis in a large cohort of patients with focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2009;24(10):3089–96. https://doi.org/10.1093/ndt/gfp229.
Hofstra JM, Lainez S, van Kuijk WH, Schoots J, Baltissen MP, Hoefsloot LH, et al. New TRPC6 gain-of-function mutation in a non-consanguineous Dutch family with late-onset focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2013;28(7):1830–8. https://doi.org/10.1093/ndt/gfs572.
Buscher AK, Konrad M, Nagel M, Witzke O, Kribben A, Hoyer PF, et al. Mutations in podocyte genes are a rare cause of primary FSGS associated with ESRD in adult patients. Clin Nephrol. 2012;78(1):47–53.
Gigante M, Caridi G, Montemurno E, Soccio M, d'Apolito M, Cerullo G, et al. TRPC6 mutations in children with steroid-resistant nephrotic syndrome and atypical phenotype. Clin J Am Soc Nephrol. 2011;6(7):1626–34. https://doi.org/10.2215/cjn.07830910.
Mir S, Yavascan O, Berdeli A, Sozeri B. TRPC6 gene variants in Turkish children with steroid-resistant nephrotic syndrome. Nephrol Dial Transplant. 2012;27(1):205–9. https://doi.org/10.1093/ndt/gfr202.
Gheissari A, Meamar R, Kheirollahi M, Rouigari M, Dehbashi M, Dehghani L, et al. TRPC6 mutational analysis in Iranian children with focal segmental glomerulosclerosis. Iran J Kidney Dis. 2018;12(6):341–9.
Zhu B, Chen N, Wang ZH, Pan XX, Ren H, Zhang W, et al. Identification and functional analysis of a novel TRPC6 mutation associated with late onset familial focal segmental glomerulosclerosis in Chinese patients. Mutat Res. 2009;664(1–2):84–90. https://doi.org/10.1016/j.mrfmmm.2008.11.021.
Toth-Manikowski S, Atta MG. Diabetic kidney disease: pathophysiology and therapeutic targets. J Diabetes Res. 2015;2015:697010. https://doi.org/10.1155/2015/697010.
Ilatovskaya DV, Palygin O, Chubinskiy-Nadezhdin V, Negulyaev YA, Ma R, Birnbaumer L, et al. Angiotensin II has acute effects on TRPC6 channels in podocytes of freshly isolated glomeruli. Kidney Int. 2014;86(3):506–14. https://doi.org/10.1038/ki.2014.71.
Anderson M, Kim EY, Hagmann H, Benzing T, Dryer SE. Opposing effects of podocin on the gating of podocyte TRPC6 channels evoked by membrane stretch or diacylglycerol. Am J Physiol Cell Physiol. 2013;305(3):C276–89. https://doi.org/10.1152/ajpcell.00095.2013.
Dietrich A, Gudermann T. TRPC6: physiological function and pathophysiological relevance. Handb Exp Pharmacol. 2014;222:157–88. https://doi.org/10.1007/978-3-642-54215-2_7.
Ilatovskaya DV, Palygin O, Levchenko V, Staruschenko A. Pharmacological characterization of the P2 receptors profile in the podocytes of the freshly isolated rat glomeruli. Am J Physiol Cell Physiol. 2013;305(10):C1050–9. https://doi.org/10.1152/ajpcell.00138.2013.
Palygin O, Ilatovskaya DV, Staruschenko A. Protease-activated receptors in kidney disease progression. Am J Physiol Renal Physiol. 2016;311(6):F1140–F4. https://doi.org/10.1152/ajprenal.00460.2016.
Roshanravan H, Dryer SE. ATP acting through P2Y receptors causes activation of podocyte TRPC6 channels: role of podocin and reactive oxygen species. Am J Physiol Renal Physiol. 2014;306(9):F1088–97. https://doi.org/10.1152/ajprenal.00661.2013.
Anderson M, Roshanravan H, Khine J, Dryer SE. Angiotensin II activation of TRPC6 channels in rat podocytes requires generation of reactive oxygen species. J Cell Physiol. 2014;229(4):434–42. https://doi.org/10.1002/jcp.24461.
Wang L, Jirka G, Rosenberg PB, Buckley AF, Gomez JA, Fields TA, et al. Gq signaling causes glomerular injury by activating TRPC6. J Clin Invest. 2015;125(5):1913–26. https://doi.org/10.1172/JCI76767.
Ilatovskaya DV, Palygin O, Levchenko V, Endres BT, Staruschenko A. The role of angiotensin II in glomerular volume dynamics and podocyte calcium handling. Sci Rep. 2017;7(1):299. https://doi.org/10.1038/s41598-017-00406-2.
Ilatovskaya DV, Levchenko V, Lowing A, Shuyskiy LS, Palygin O, Staruschenko A. Podocyte injury in diabetic nephropathy: implications of angiotensin II-dependent activation of TRPC channels. Sci Rep. 2015;5:17637. https://doi.org/10.1038/srep17637.
Eckel J, Lavin PJ, Finch EA, Mukerji N, Burch J, Gbadegesin R, et al. TRPC6 enhances angiotensin II-induced albuminuria. J Am Soc Nephrol. 2011;22(3):526–35.
Tian D, Jacobo SM, Billing D, Rozkalne A, Gage SD, Anagnostou T, et al. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci Signal. 2010;3(145):ra77.
Sonneveld R, van der Vlag J, Baltissen MP, Verkaart SA, Wetzels JF, Berden JH, et al. Glucose specifically regulates TRPC6 expression in the podocyte in an AngII-dependent manner. Am J Pathol. 2014;184(6):1715–26. https://doi.org/10.1016/j.ajpath.2014.02.008.
Nijenhuis T, Sloan AJ, Hoenderop JG, Flesche J, van Goor H, Kistler AD, et al. Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am J Pathol. 2011;179(4):1719–32. https://doi.org/10.1016/j.ajpath.2011.06.033.
Zhang H, Ding J, Fan Q, Liu S. TRPC6 up-regulation in Ang II-induced podocyte apoptosis might result from ERK activation and NF-kappaB translocation. Exp Biol Med. 2009;234(9):1029–36.
Hayek SS, Koh KH, Grams ME, Wei C, Ko YA, Li J, et al. A tripartite complex of suPAR, APOL1 risk variants and alphavbeta3 integrin on podocytes mediates chronic kidney disease. Nat Med. 2017;23(8):945–53. https://doi.org/10.1038/nm.4362.
Rinschen MM, Huesgen PF, Koch RE. The podocyte protease web: uncovering the gatekeepers of glomerular disease. Am J Physiol Renal Physiol. 2018;315(6):F1812-F1816.. https://doi.org/10.1152/ajprenal.00380.2018.
Kim EY, Roshanravan H, Dryer SE. Changes in podocyte TRPC channels evoked by plasma and sera from patients with recurrent FSGS and by putative glomerular permeability factors. Biochim Biophys Acta. 2017;1863(9):2342–54. https://doi.org/10.1016/j.bbadis.2017.06.010.
Kim EY, Hassanzadeh Khayyat N, Dryer SE. Mechanisms underlying modulation of podocyte TRPC6 channels by suPAR: role of NADPH oxidases and Src family tyrosine kinases. Biochim Biophys Acta Mol Basis Dis. 2018;1864(10):3527–36. https://doi.org/10.1016/j.bbadis.2018.08.007.
Dande RR, Peev V, Altintas MM, Reiser J. Soluble urokinase receptor and the kidney response in diabetes mellitus. J Diabetes Res. 2017;2017:3232848. https://doi.org/10.1155/2017/3232848.
Hayek SS, Sever S, Ko Y-A, Trachtman H, Awad M, Wadhwani S, et al. Soluble urokinase receptor and chronic kidney disease. N Engl J Med. 2015;373(20):1916–25. https://doi.org/10.1056/NEJMoa1506362.
Guthoff M, Wagner R, Randrianarisoa E, Hatziagelaki E, Peter A, Häring H-U, et al. Soluble urokinase receptor (suPAR) predicts microalbuminuria in patients at risk for type 2 diabetes mellitus. Sci Rep. 2017;7:40627. https://doi.org/10.1038/srep40627.
Guan Y, Nakano D, Zhang Y, Li L, Liu W, Nishida M, et al. A protease-activated receptor-1 antagonist protects against podocyte injury in a mouse model of nephropathy. J Pharmacol Sci. 2017. https://doi.org/10.1016/j.jphs.2017.09.002.
Waasdorp M, Duitman J, Florquin S, Spek CA. Vorapaxar treatment reduces mesangial expansion in streptozotocin-induced diabetic nephropathy in mice. Oncotarget. 2018;9(31):21655–62. https://doi.org/10.18632/oncotarget.25069.
Waasdorp M, Duitman J, Florquin S, Spek CA. Protease-activated receptor-1 deficiency protects against streptozotocin-induced diabetic nephropathy in mice. Sci Rep. 2016;6:33030. https://doi.org/10.1038/srep33030.
Kitamoto Y, Tomita K, Imamura T. Assessment of thrombin in the urine of glomerulonephritic patients by enzyme-linked immunosorbent assay. Ann Clin Biochem. 2004;41(Pt 2):133–7. https://doi.org/10.1258/000456304322880023.
Bauer KA, Weiss LM, Sparrow D, Vokonas PS, Rosenberg RD. Aging-associated changes in indices of thrombin generation and protein C activation in humans. Normative Aging Study. J Clin Invest. 1987;80(6):1527–34. https://doi.org/10.1172/JCI113238.
Konieczynska M, Fil K, Bazanek M, Undas A. Prolonged duration of type 2 diabetes is associated with increased thrombin generation, prothrombotic fibrin clot phenotype and impaired fibrinolysis. Thromb Haemost. 2014;111(4):685–93. https://doi.org/10.1160/th13-07-0566.
Palygin O, Ilatovskaya DV, Levchenko V, Klemens CA, Dissanayake L, Williams AM, et al. Characterization of purinergic receptor expression in ARPKD cystic epithelia. Purinergic Signal. 2018. https://doi.org/10.1007/s11302-018-9632-5.
Marko L, Mannaa M, Haschler TN, Kramer S, Gollasch M. Renoprotection: focus on TRPV1, TRPV4, TRPC6 and TRPM2. Acta Physiol. 2017;219(3):589–612. https://doi.org/10.1111/apha.12828.
•• Ilatovskaya DV, Blass G, Palygin O, Levchenko V, Pavlov TS, Grzybowski MN, et al. A NOX4/TRPC6 pathway in podocyte calcium regulation and renal damage in diabetic kidney disease. J Am Soc Nephrol. 2018;29(7):1917–27. https://doi.org/10.1681/ASN.2018030280 This study demostrate that H 2 O 2 stimulates calcium influx via TRPC6 channels, and podocytes isolated from TRPC6-knockout mice are protected from injury induced by H 2 O 2 . Furthermore, diabetes-induced increase in basal and Ang II–elicited calcium flux in podocytes was blunted in STZ-SS Nox4-/- rats.
Jha JC, Banal C, Okabe J, Gray SP, Hettige T, Chow BSM, et al. NADPH oxidase Nox5 accelerates renal injury in diabetic nephropathy. Diabetes. 2017;66(10):2691–703. https://doi.org/10.2337/db16-1585.
Graham S, Gorin Y, Abboud HE, Ding M, Lee DY, Shi H, et al. Abundance of TRPC6 protein in glomerular mesangial cells is decreased by ROS and PKC in diabetes. Am J Physiol Cell Physiol. 2011;301(2):C304–15. https://doi.org/10.1152/ajpcell.00014.2011.
Chiluiza D, Krishna S, Schumacher VA, Schlondorff J. Gain-of-function mutations in transient receptor potential C6 (TRPC6) activate extracellular signal-regulated kinases 1/2 (ERK1/2). J Biol Chem. 2013;288(25):18407–20. https://doi.org/10.1074/jbc.M113.463059.
Azumaya CM, Sierra-Valdez F, Cordero-Morales JF, Nakagawa T. Cryo-EM structure of the cytoplasmic domain of murine transient receptor potential cation channel subfamily C member 6 (TRPC6). J Biol Chem. 2018;293(26):10381–91. https://doi.org/10.1074/jbc.RA118.003183.
•• Tang Q, Guo W, Zheng L, Wu JX, Liu M, Zhou X, et al. Structure of the receptor-activated human TRPC6 and TRPC3 ion channels. Cell Res. 2018;28(7):746–55. https://doi.org/10.1038/s41422-018-0038-2 This is the first description of the structure of human TRPC6 homotetramer.
Staruschenko A. TRPC6 in diabetic kidney disease: good guy or bad guy? Kidney Int. 2019;95(2):256–8. https://doi.org/10.1016/j.kint.2018.10.027.
Rudemiller NP, Mattson DL. Candidate genes for hypertension: insights from the Dahl S rat. Am J Physiol Renal Physiol. 2015;309(12):F993–5. https://doi.org/10.1152/ajprenal.00092.2015.
Pavlov TS, Levchenko V, O'Connor PM, Ilatovskaya DV, Palygin O, Mori T, et al. Deficiency of renal cortical EGF increases ENaC activity and contributes to salt-sensitive hypertension. J Am Soc Nephrol. 2013;24(7):1053–62. https://doi.org/10.1681/ASN.2012080839.
Feng D, Yang C, Geurts A, Kurth T, Liang M, Lazar J, et al. Increased expression of NAD(P)H oxidase subunit p67 phox in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metab. 2012;15:201–8.
Slaughter TN, Paige A, Spires D, Kojima N, Kyle PB, Garrett MR, et al. Characterization of the development of renal injury in Type-1 diabetic Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol. 2013;305(7):R727–34. https://doi.org/10.1152/ajpregu.00382.2012.
Spires D, Ilatovskaya DV, Levchenko V, North PE, Geurts AM, Palygin O, et al. Protective role of Trpc6 knockout in the progression of diabetic kidney disease. Am J Physiol Renal Physiol. 2018;315(4):F1091–F7. https://doi.org/10.1152/ajprenal.00155.2018.
Khayyat NH, Kim EY, Dryer SE. TRPC6 inactivation does not protect against diabetic kidney disease in streptozotocin (STZ)-treated Sprague-Dawley rats. FASEB J. 2019;33(1_supplement):567.3 (Abstract:851.9)). https://doi.org/10.1096/fasebj.2019.33.1_supplement.567.3.
• Kim EY, Yazdizadeh Shotorbani P, Dryer SE. Trpc6 inactivation confers protection in a model of severe nephrosis in rats. J Mol Med. 2018;96(7):631–44. https://doi.org/10.1007/s00109-018-1648-3 This study revealed that genetic inactivation of TRPC6 in Sprague-Dawley rats markedly reduced albuminuria, glomerulosclerosis, and ultrastructural changes in glomeruli that occurred during the chronic phase of PAN nephrosis.
Brosius FC, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, et al. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2009;20(12):2503–12. https://doi.org/10.1681/asn.2009070721.
Wang L, Chang JH, Buckley AF, Spurney RF. Knockout of TRPC6 promotes insulin resistance and exacerbates glomerular injury in Akita mice. Kidney Int. 2019;95(2):321–32. https://doi.org/10.1016/j.kint.2018.09.026.
Wang Y, Jarad G, Tripathi P, Pan M, Cunningham J, Martin DR, et al. Activation of NFAT signaling in podocytes causes glomerulosclerosis. J Am Soc Nephrol. 2010;21(10):1657–66. https://doi.org/10.1681/asn.2009121253.
Schlondorff J, del Camino D, Carrasquillo R, Lacey V, Pollak MR. TRPC6 mutations associated with focal segmental glomerulosclerosis cause constitutive activation of NFAT-dependent transcription. Am J Physiol Cell Physiol. 2009;296(3):C558–C69.
Ma R, Xu Y, Zhou H, Zhang D, Yao D, Song L, et al. Participation of the AngII/TRPC6/NFAT axis in the pathogenesis of podocyte injury in rats with type 2 diabetes. Mol Med Rep. 2019;19(3):2421–30. https://doi.org/10.3892/mmr.2019.9871.
Ma R, Liu L, Jiang W, Yu Y, Song H. FK506 ameliorates podocyte injury in type 2 diabetic nephropathy by down-regulating TRPC6 and NFAT expression. Int J Clin Exp Pathol. 2015;8(11):14063–74.
Fu Y, Wang C, Zhang D, Xin Y, Li J, Zhang Y, et al. Increased TRPC6 expression is associated with tubular epithelial cell proliferation and inflammation in diabetic nephropathy. Mol Immunol. 2018;94:75–81. https://doi.org/10.1016/j.molimm.2017.12.014.
Rajani R, Pastor-Soler NM, Hallows KR. Role of AMP-activated protein kinase in kidney tubular transport, metabolism, and disease. Curr Opin Nephrol Hypertens. 2017;26(5):375–83. https://doi.org/10.1097/mnh.0000000000000349.
Rachubik P, Szrejder M, Rogacka D, Audzeyenka I, Rychlowski M, Angielski S, et al. The TRPC6-AMPK pathway is involved in insulin-dependent cytoskeleton reorganization and glucose uptake in cultured rat podocytes. Cell Physiol Biochem. 2018;51(1):393–410. https://doi.org/10.1159/000495236.
Ji T, Zhang C, Ma L, Wang Q, Zou L, Meng K, et al. TRPC6-mediated Ca2+ signaling is required for hypoxia-induced autophagy in human podocytes. Cell Physiol Biochem. 2018;48(4):1782–92. https://doi.org/10.1159/000492351.
Huang H, You Y, Lin X, Tang C, Gu X, Huang M, et al. Inhibition of TRPC6 signal pathway alleviates podocyte injury induced by TGF-beta1. Cell Physiol Biochem. 2017;41(1):163–72. https://doi.org/10.1159/000455985.
Yu L, Lin Q, Liao H, Feng J, Dong X, Ye J. TGF-beta1 induces podocyte injury through Smad3-ERK-NF-kappaB pathway and Fyn-dependent TRPC6 phosphorylation. Cell Physiol Biochem. 2010;26(6):869–78. https://doi.org/10.1159/000323996.
Liu B, He X, Li S, Xu B, Birnbaumer L, Liao Y. Deletion of diacylglycerol-responsive TRPC genes attenuates diabetic nephropathy by inhibiting activation of the TGFbeta1 signaling pathway. Am J Transl Res. 2017;9(12):5619–30.
• Lin BL, Matera D, Doerner JF, Zheng N, Del Camino D, Mishra S, et al. In vivo selective inhibition of TRPC6 by antagonist BI 749327 ameliorates fibrosis and dysfunction in cardiac and renal disease. Proc Natl Acad Sci U S A. 2019. https://doi.org/10.1073/pnas.1815354116 In this study, newly developed orally bioavailable TRPC6-specific inhibitor, BI 749327, revealed its capacity to improve cardiac function in the context of abnormal hemodynamic stress. Similarly, BI 749327 suppressed myofibroblast activation and fibrosis in a renal disease model.
Kim EY, Shotorbani PY, Dryer SE. TRPC6 inactivation does not affect loss of renal function in nephrotoxic serum glomerulonephritis in rats, but reduces severity of glomerular lesions. Biochem Biophys Rep. 2019;17:139–50. https://doi.org/10.1016/j.bbrep.2018.12.006.
Li W, Ding Y, Smedley C, Wang Y, Chaudhari S, Birnbaumer L, et al. Increased glomerular filtration rate and impaired contractile function of mesangial cells in TRPC6 knockout mice. Sci Rep. 2017;7(1):4145. https://doi.org/10.1038/s41598-017-04067-z.
Wu YL, Xie J, An SW, Oliver N, Barrezueta NX, Lin MH, et al. Inhibition of TRPC6 channels ameliorates renal fibrosis and contributes to renal protection by soluble klotho. Kidney Int. 2017;91(4):830–41. https://doi.org/10.1016/j.kint.2016.09.039.
Hofstra JM, Coenen MJ, Schijvenaars MM, Berden JH, van der Vlag J, Hoefsloot LH, et al. TRPC6 single nucleotide polymorphisms and progression of idiopathic membranous nephropathy. PLoS One. 2014;9(7):e102065. https://doi.org/10.1371/journal.pone.0102065.
Moller CC, Wei C, Altintas MM, Li J, Greka A, Ohse T, et al. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol. 2007;18(1):29–36.
Chen Y, Lin L, Tao X, Song Y, Cui J, Wan J. The role of podocyte damage in the etiology of ischemia-reperfusion acute kidney injury and post-injury fibrosis. BMC Nephrol. 2019;20(1):106. https://doi.org/10.1186/s12882-019-1298-x.
Yu Y, Zhang L, Xu G, Wu Z, Li Q, Gu Y, et al. Angiotensin II type I receptor agonistic autoantibody induces podocyte injury via activation of the TRPC6- calcium/calcineurin pathway in pre-eclampsia. Kidney Blood Press Res. 2018;43(5):1666–76. https://doi.org/10.1159/000494744.
Funding
Research in the authors’ laboratories is supported by the American Heart Association 16EIA26720006, 17SDG33660149, and 18PRE34030127, the National Heart, Lung, and Blood Institute R35 HL135749, and the Department of Veteran Affairs I01 BX004024.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Mechanisms of Hypertension and Target-Organ Damage
Rights and permissions
About this article
Cite this article
Staruschenko, A., Spires, D. & Palygin, O. Role of TRPC6 in Progression of Diabetic Kidney Disease. Curr Hypertens Rep 21, 48 (2019). https://doi.org/10.1007/s11906-019-0960-9
Published:
DOI: https://doi.org/10.1007/s11906-019-0960-9