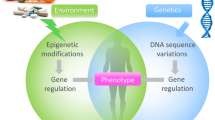
Abstract
Hypertension (HT) is among the major components of the metabolic syndrome, i.e., obesity, dyslipidemia, and hyperglycemia/insulin resistance. It represents a significant health problem with foremost risks for chronic cardiovascular disease and a significant cause of morbidity and mortality worldwide. Therefore, it is not surprising that this disorder constitutes a serious public health concern. Although multiple studies have stressed the multifactorial nature of HT, the pathogenesis remains largely unknown. However, if we want to reduce the global prevalence of HT, restrain the number of deaths (currently 9.4 million/year in the world), and alleviate the socio-economic burden, a deeper insight into the mechanisms is urgently needed in order to define new meaningful therapeutic targets. Recently, the role of epigenetics in the development of various complex diseases has attracted much attention. In the present review, we provide a critical update on the available literature and ongoing research regarding the epigenetic modifications of genes involved in several pathways of elevated blood pressure, especially those linked to the vascular epithelium. This review also focuses on the role of microRNA (miRNA) in the regulation of gene expression associated with HT and of fetal programming mediating susceptibility to HT in adulthood.



Similar content being viewed by others
Abbreviations
- ACE:
-
Angiotensin-converting enzyme
- AGTR1:
-
Angiotensin II receptor type 1
- Ang:
-
Angiotensin
- CMV:
-
Cytomegalovirus
- CNS:
-
Central nervous system
- Dot-1:
-
Disruptor of telomere silencing 1
- ENaC:
-
Epithelial of sodium channel
- eNOS:
-
Endothelial nitric oxide synthase
- H3K4:
-
Histone H3 lysine 4
- HT:
-
Hypertension
- miRNA:
-
MicroRNA
- NET:
-
Noradrenaline transporter
- OxS:
-
Oxidative stress
- RAAS:
-
Renin–angiotensin–aldosterone system
- ROS:
-
Reactive oxygen species
- SMC:
-
Smooth muscle cells
- SNP:
-
Single nucleotide polymorphism
- SOD:
-
Superoxide dismutase
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Carretero OA, Oparil S. Essential hypertension. Circulation. 2000;101(3):329–35.
Simon PH, Sylvestre M-P, Tremblay J, Hamet P. Key considerations and methods in the study of gene–environment interactions. Am J Hypertens. 2016;29(8):891–9.
Cat AND, Montezano AC, Touyz RM. Renin–angiotensin–aldosterone system: new concepts. Hypertension. 2013:84–100.
Wei LK, Au A, Teh LK, Lye HS. Recent advances in the genetics of hypertension. 2016.
• Baubec T, Schübeler D. Genomic patterns and context specific interpretation of DNA methylation. Curr Opin Genet Dev. 2014;25:85–92. The review focuses on the link between DNA methylation and the potential function of epigenetics.
Arnsdorf EJ, Tummala P, Castillo AB, Zhang F, Jacobs CR. The epigenetic mechanism of mechanically induced osteogenic differentiation. J Biomech. 2010;43(15):2881–6.
Lee RC, Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science. 2001;294(5543):862–4.
Yoo AS, Staahl BT, Chen L, Crabtree GR. MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature. 2009;460(7255):642–6.
Floren M, Bonani W, Dharmarajan A, Motta A, Migliaresi C, Tan W. Human mesenchymal stem cells cultured on silk hydrogels with variable stiffness and growth factor differentiate into mature smooth muscle cell phenotype. Acta Biomater. 2016;31:156–66. doi:10.1016/j.actbio.2015.11.051.
Byon CH, Heath JM, Chen Y. Redox signaling in cardiovascular pathophysiology: a focus on hydrogen peroxide and vascular smooth muscle cells. Redox Biol. 2016;9:244–53. doi:10.1016/j.redox.2016.08.015.
• Liu R, Leslie KL, Martin KA. Epigenetic regulation of smooth muscle cell plasticity. Biochimica et Biophysica Acta (BBA)-Gene Regulatory Mechanisms. 2015;1849(4):448–53. The review highlights the importance of how the smooth muscle cells can be affected by the epigenetic changes across the genome.
•• Manabe I, Owens GK. Recruitment of serum response factor and hyperacetylation of histones at smooth muscle–specific regulatory regions during differentiation of a novel p19-derived in vitro smooth muscle differentiation system. Circ Res. 2001;88(11):1127–34. In vitro studies on mechanisms controlling SMC differentiation marker genes within chromatin.
Cao D, Wang Z, Zhang C-L, Oh J, Xing W, Li S, et al. Modulation of smooth muscle gene expression by association of histone acetyltransferases and deacetylases with myocardin. Mol Cell Biol. 2005;25(1):364–76.
Misárková E, Behuliak M, Bencze M, Zicha J. Excitation-contraction coupling and excitation-transcription coupling in blood vessels: their possible interactions in hypertensive vascular remodeling. Physiol Res. 2016;65(2):173.
Qin X, Wang X, Wang Y, Tang Z, Cui Q, Xi J, et al. MicroRNA-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells. Proc Natl Acad Sci U S A. 2010;107(7):3240–4. doi:10.1073/pnas.0914882107.
Kohlstedt K, Trouvain C, Boettger T, Shi L, Fisslthaler B, Fleming I. AMP-activated protein kinase regulates endothelial cell angiotensin-converting enzyme expression via p53 and the post-transcriptional regulation of microRNA-143/145. Circ Res. 2013;112(8):1150–8. doi:10.1161/CIRCRESAHA.113.301282.
Davies PF, Manduchi E, Stoeckert CJ, Jiménez JM, Jiang Y-Z. Emerging topic: flow-related epigenetic regulation of endothelial phenotype through DNA methylation. Vasc Pharmacol. 2014;62(2):88–93.
Guay SP, Brisson D, Lamarche B, Biron S, Lescelleur O, Biertho L, et al. ADRB3 gene promoter DNA methylation in blood and visceral adipose tissue is associated with metabolic disturbances in men. Epigenomics. 2014;6(1):33–43. doi:10.2217/epi.13.82.
Wang F, Demura M, Cheng Y, Zhu A, Karashima S, Yoneda T, et al. Dynamic CCAAT/enhancer binding protein-associated changes of DNA methylation in the angiotensinogen gene. Hypertension. 2014;63(2):281–8. doi:10.1161/HYPERTENSIONAHA.113.02303.
Campion J, Milagro FI, Martinez JA. Individuality and epigenetics in obesity. Obes Rev. 2009;10(4):383–92. doi:10.1111/j.1467-789X.2009.00595.x.
• Coats A, Jain S. Protective effects of nebivolol from oxidative stress to prevent hypertension-related target organ damage. J Hum Hypertens. 2017; doi:10.1038/jhh.2017.8. Development of targeted therapies related to oxidative stress in hypertensive patients.
Ilatovskaya DV, Pavlov TS, Levchenko V, Staruschenko A. ROS production as a common mechanism of ENaC regulation by EGF, insulin, and IGF-1. Am J Physiol Cell Physiol. 2013;304(1):C102–11. doi:10.1152/ajpcell.00231.2012.
Chia N, Wang L, Lu X, Senut MC, Brenner C, Ruden DM. Hypothesis: environmental regulation of 5-hydroxymethylcytosine by oxidative stress. Epigenetics. 2011;6(7):853–6.
Archer SL. Acquired mitochondrial abnormalities, including epigenetic inhibition of superoxide dismutase 2, in pulmonary hypertension and cancer: therapeutic implications. Adv Exp Med Biol. 2016;903:29–53. doi:10.1007/978-1-4899-7678-9_3.
Nozik-Grayck E, Woods C, Stearman RS, Venkataraman S, Ferguson BS, Swain K, et al. Histone deacetylation contributes to low extracellular superoxide dismutase expression in human idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2016;311(1):L124–34. doi:10.1152/ajplung.00263.2015.
• Hirooka Y, Kishi T, Ito K, Sunagawa K. Potential clinical application of recently discovered brain mechanisms involved in hypertension. Hypertension. 2013;62(6):995–1002. doi:10.1161/HYPERTENSIONAHA.113.00801. The review highlights a better knowledge on the sympathetic nervous system in hypertension.
Esler M, Alvarenga M, Pier C, Richards J, El-Osta A, Barton D, et al. The neuronal noradrenaline transporter, anxiety and cardiovascular disease. J Psychopharmacol. 2006;20(4 Suppl):60–6. doi:10.1177/1359786806066055.
Esler M, Eikelis N, Schlaich M, Lambert G, Alvarenga M, Kaye D, et al. Human sympathetic nerve biology: parallel influences of stress and epigenetics in essential hypertension and panic disorder. Ann N Y Acad Sci. 2008;1148:338–48. doi:10.1196/annals.1410.064.
Marques FZ, Campain AE, Tomaszewski M, Zukowska-Szczechowska E, Yang YH, Charchar FJ, et al. Gene expression profiling reveals renin mRNA overexpression in human hypertensive kidneys and a role for microRNAs. Hypertension. 2011;58(6):1093–8. doi:10.1161/HYPERTENSIONAHA.111.180729.
Hultstrom M. Development of structural kidney damage in spontaneously hypertensive rats. J Hypertens. 2012;30(6):1087–91. doi:10.1097/HJH.0b013e328352b89a.
Dobrian AD, Schriver SD, Prewitt RL. Role of angiotensin II and free radicals in blood pressure regulation in a rat model of renal hypertension. Hypertension. 2001;38(3):361–6.
Zhang D, Yu ZY, Cruz P, Kong Q, Li S, Kone BC. Epigenetics and the control of epithelial sodium channel expression in collecting duct. Kidney Int. 2009;75(3):260–7. doi:10.1038/ki.2008.475.
Nishimoto M, Fujita T. Renal mechanisms of salt-sensitive hypertension: contribution of two steroid receptor-associated pathways. Am J Physiol Renal Physiol. 2015;308(5):F377–87. doi:10.1152/ajprenal.00477.2013.
Oudit GY, Penninger JM. Cardiac regulation by phosphoinositide 3-kinases and PTEN. Cardiovasc Res. 2009;82(2):250–60. doi:10.1093/cvr/cvp014.
Kang H, Chang W, Hurley M, Vignery A, Wu D. Important roles of PI3Kgamma in osteoclastogenesis and bone homeostasis. Proc Natl Acad Sci U S A. 2010;107(29):12901–6. doi:10.1073/pnas.1001499107.
Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2(7):247–57. doi:10.1002/emmm.201000080.
Weber G, Pushpakumar S, Sen U. Hydrogen sulfide alleviates hypertensive kidney dysfunction through an epigenetic mechanism. Am J Physiol Heart Circ Physiol. 2017; doi:10.1152/ajpheart.00637.2016.
Arpon A, Riezu-Boj JI, Milagro FI, Razquin C, Martinez-Gonzalez MA, Corella D, et al. Adherence to Mediterranean diet is associated with methylation changes in inflammation-related genes in peripheral blood cells. J Physiol Biochem. 2017; doi:10.1007/s13105-017-0552-6.
Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10(7):505–14. doi:10.1038/nrc2868.
•• Henning RJ. Therapeutic angiogenesis: angiogenic growth factors for ischemic heart disease. Futur Cardiol. 2016;12(5):585–99. doi:10.2217/fca-2016-0006. A better knowledge on therapeutic angiogenesis.
Marek-Trzonkowska N, Kwieczynska A, Reiwer-Gostomska M, Kolinski T, Molisz A, Siebert J. Arterial hypertension is characterized by imbalance of pro-angiogenic versus anti-angiogenic factors. PLoS One. 2015;10(5):e0126190. doi:10.1371/journal.pone.0126190.
Kontaraki JE, Marketou ME, Zacharis EA, Parthenakis FI, Vardas PE. MicroRNA-9 and microRNA-126 expression levels in patients with essential hypertension: potential markers of target-organ damage. J Am Soc Hypertens. 2014;8(6):368–75. doi:10.1016/j.jash.2014.03.324.
Li S, Zhu J, Zhang W, Chen Y, Zhang K, Popescu LM, et al. Signature microRNA expression profile of essential hypertension and its novel link to human cytomegalovirus infection. Circulation. 2011;124(2):175–84. doi:10.1161/CIRCULATIONAHA.110.012237.
• Kontaraki JE, Marketou ME, Parthenakis FI, Maragkoudakis S, Zacharis EA, Petousis S, et al. Hypertrophic and antihypertrophic microRNA levels in peripheral blood mononuclear cells and their relationship to left ventricular hypertrophy in patients with essential hypertension. J Am Soc Hypertens. 2015;9(10):802–10. doi:10.1016/j.jash.2015.07.013. MicroRNAs regulate several pathophysiological cardiovascular diseases promoting beneficial therapeutic strategies.
•• Kontaraki JE, Marketou ME, Zacharis EA, Parthenakis FI, Vardas PE. Differential expression of vascular smooth muscle-modulating microRNAs in human peripheral blood mononuclear cells: novel targets in essential hypertension. J Hum Hypertens. 2014;28(8):510–6. doi:10.1038/jhh.2013.117. MicroRNAs regulating hypertension in vascular smooth muscle
Rhodes CJ, Wharton J, Boon RA, Roexe T, Tsang H, Wojciak-Stothard B, et al. Reduced microRNA-150 is associated with poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;187(3):294–302. doi:10.1164/rccm.201205-0839OC.
Courboulin A, Paulin R, Giguere NJ, Saksouk N, Perreault T, Meloche J, et al. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med. 2011;208(3):535–48. doi:10.1084/jem.20101812.
Zhu N, Zhang D, Chen S, Liu X, Lin L, Huang X, et al. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis. 2011;215(2):286–93. doi:10.1016/j.atherosclerosis.2010.12.024.
Eskildsen TV, Jeppesen PL, Schneider M, Nossent AY, Sandberg MB, Hansen PB, et al. Angiotensin II regulates microRNA-132/-212 in hypertensive rats and humans. Int J Mol Sci. 2013;14(6):11190–207. doi:10.3390/ijms140611190.
Kumar S, Kim CW, Simmons RD, Jo H. Role of flow-sensitive microRNAs in endothelial dysfunction and atherosclerosis: mechanosensitive athero-miRs. Arterioscler Thromb Vasc Biol. 2014;34(10):2206–16. doi:10.1161/ATVBAHA.114.303425.
Harris TA, Yamakuchi M, Kondo M, Oettgen P, Lowenstein CJ. Ets-1 and Ets-2 regulate the expression of microRNA-126 in endothelial cells. Arterioscler Thromb Vasc Biol. 2010;30(10):1990–7. doi:10.1161/ATVBAHA.110.211706.
Cheng HS, Sivachandran N, Lau A, Boudreau E, Zhao JL, Baltimore D, et al. MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol Med. 2013;5(7):1017–34. doi:10.1002/emmm.201202318.
Yang Y, Zhou Y, Cao Z, Tong XZ, Xie HQ, Luo T, et al. miR-155 functions downstream of angiotensin II receptor subtype 1 and calcineurin to regulate cardiac hypertrophy. Exp Ther Med. 2016;12(3):1556–62. doi:10.3892/etm.2016.3506.
•• Karolina DS, Tavintharan S, Armugam A, Sepramaniam S, Pek SL, Wong MT, et al. Circulating miRNA profiles in patients with metabolic syndrome. J Clin Endocrinol Metab. 2012;97(12):E2271–6. doi:10.1210/jc.2012-1996. Implication of circulating microRNAs in metabolic syndrome.
Wei C, Henderson H, Spradley C, Li L, Kim IK, Kumar S, et al. Circulating miRNAs as potential marker for pulmonary hypertension. PLoS One. 2013;8(5):e64396. doi:10.1371/journal.pone.0064396.
Cengiz M, Karatas OF, Koparir E, Yavuzer S, Ali C, Yavuzer H, et al. Differential expression of hypertension-associated microRNAs in the plasma of patients with white coat hypertension. Medicine (Baltimore). 2015;94(13):e693. doi:10.1097/MD.0000000000000693.
Park MY, Herrmann SM, Saad A, Widmer RJ, Tang H, Zhu XY, et al. Circulating and renal vein levels of microRNAs in patients with renal artery stenosis. Nephrol Dial Transplant. 2015;30(3):480–90. doi:10.1093/ndt/gfu341.
Marques FZ, Romaine SP, Denniff M, Eales J, Dormer J, Garrelds IM, et al. Signatures of miR-181a on renal transcriptome and blood pressure. Mol Med. 2015; doi:10.2119/molmed.2015.00096.
Mandraffino G, Imbalzano E, Sardo MA, D'Ascola A, Mamone F, Lo Gullo A, et al. Circulating progenitor cells in hypertensive patients with different degrees of cardiovascular involvement. J Hum Hypertens. 2014;28(9):543–50. doi:10.1038/jhh.2014.7.
• Yang Q, Jia C, Wang P, Xiong M, Cui J, Li L, et al. MicroRNA-505 identified from patients with essential hypertension impairs endothelial cell migration and tube formation. Int J Cardiol. 2014;177(3):925–34. doi:10.1016/j.ijcard.2014.09.204. Role of circulating miRNA on angiogenesis.
Thulasingam S, Massilamany C, Gangaplara A, Dai H, Yarbaeva S, Subramaniam S, et al. miR-27b*, an oxidative stress-responsive microRNA modulates nuclear factor-kB pathway in RAW 264.7 cells. Mol Cell Biochem. 2011;352(1–2):181–8. doi:10.1007/s11010-011-0752-2.
Zhang W, Yan L, Li Y, Chen W, Hu N, Wang H, et al. Roles of miRNA-24 in regulating endothelial nitric oxide synthase expression and vascular endothelial cell proliferation. Mol Cell Biochem. 2015;405(1–2):281–9. doi:10.1007/s11010-015-2418-y.
Sun HX, Zeng DY, Li RT, Pang RP, Yang H, Hu YL, et al. Essential role of microRNA-155 in regulating endothelium-dependent vasorelaxation by targeting endothelial nitric oxide synthase. Hypertension. 2012;60(6):1407–14. doi:10.1161/HYPERTENSIONAHA.112.197301.
Magenta A, Greco S, Gaetano C, Martelli F. Oxidative stress and microRNAs in vascular diseases. Int J Mol Sci. 2013;14(9):17319–46. doi:10.3390/ijms140917319.
Wang L, Yuan Y, Li J, Ren H, Cai Q, Chen X, et al. MicroRNA-1 aggravates cardiac oxidative stress by post-transcriptional modification of the antioxidant network. Cell Stress Chaperones. 2015;20(3):411–20. doi:10.1007/s12192-014-0565-9.
Hulsmans M, Holvoet P. The vicious circle between oxidative stress and inflammation in atherosclerosis. J Cell Mol Med. 2010;14(1–2):70–8. doi:10.1111/j.1582-4934.2009.00978.x.
Celic T, Metzinger-Le Meuth V, Six I, Massy ZA, Metzinger L. The mir-221/222 cluster is a key player in vascular biology via the fine-tuning of endothelial cell physiology. Curr Vasc Pharmacol. 2017;15(1):40–6.
Santovito D, Mandolini C, Marcantonio P, De Nardis V, Bucci M, Paganelli C, et al. Overexpression of microRNA-145 in atherosclerotic plaques from hypertensive patients. Expert Opin Ther Targets. 2013;17(3):217–23. doi:10.1517/14728222.2013.745512.
Li N, Hwangbo C, Jaba IM, Zhang J, Papangeli I, Han J, et al. miR-182 modulates myocardial hypertrophic response induced by angiogenesis in heart. Sci Rep. 2016;6:21228. doi:10.1038/srep21228.
Nicoli S, Standley C, Walker P, Hurlstone A, Fogarty KE, Lawson ND. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature. 2010;464(7292):1196–200. doi:10.1038/nature08889.
Otsuka M, Zheng M, Hayashi M, Lee JD, Yoshino O, Lin S, et al. Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J Clin Invest. 2008;118(5):1944–54. doi:10.1172/JCI33680.
Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324(5935):1710–3. doi:10.1126/science.1174381.
•• Kato N, Loh M, Takeuchi F, Verweij N, Wang X, Zhang W, et al. Trans-ancestry genome-wide association study identifies 12 genetic loci influencing blood pressure and implicates a role for DNA methylation. Nat Genet. 2015;47(11):1282–93. Role of DNA methylation in blood pressure regulation.
Raftopoulos L, Katsi V, Makris T, Tousoulis D, Stefanadis C, Kallikazaros I. Epigenetics, the missing link in hypertension. Life Sci. 2015;129:22–6.
Udali S, Guarini P, Moruzzi S, Choi SW, Friso S. Cardiovascular epigenetics: from DNA methylation to microRNAs. Mol Asp Med. 2013;34(4):883–901. doi:10.1016/j.mam.2012.08.001.
Friso S, Carvajal CA, Fardella CE, Olivieri O. Epigenetics and arterial hypertension: the challenge of emerging evidence. Transl Res. 2015;165(1):154–165. doi:10.1016/j.trsl.2014.06.007.
Bavishi C, Bangalore S, Messerli FH. Renin angiotensin aldosterone system inhibitors in hypertension: is there evidence for benefit independent of blood pressure reduction? Prog Cardiovasc Dis. 2016;59(3):253–61. doi:10.1016/j.pcad.2016.10.002.
Pei F, Wang X, Yue R, Chen C, Huang J, Huang J, et al. Differential expression and DNA methylation of angiotensin type 1A receptors in vascular tissues during genetic hypertension development. Mol Cell Biochem. 2015;402(1–2):1–8. doi:10.1007/s11010-014-2295-9.
De Vries N, Prestes P, Rana I, Harrap SB, Charchar FJ. YIA 03-04 epigenetic changes after acute treatment with acute angiotensin converting enzyme inhibitors (ACEi). J Hypertens. 2016;34(Suppl 1 - ISH 2016 Abstract Book):e204–e5. doi:10.1097/01.hjh.0000500443.76719.d5.
Chu A, Gozal D, Cortese R, Wang Y. Cardiovascular dysfunction in adult mice following postnatal intermittent hypoxia. Pediatr Res. 2015;77(3):425–33. doi:10.1038/pr.2014.197.
Ast J, Jablecka A, Bogdanski P, Smolarek I, Krauss H, Chmara E. Evaluation of the antihypertensive effect of L-arginine supplementation in patients with mild hypertension assessed with ambulatory blood pressure monitoring. Med Sci Monit. 2010;16(5):CR266–71.
Seckl JR, Meaney MJ. Glucocorticoid programming. Ann N Y Acad Sci. 2004;1032:63–84. doi:10.1196/annals.1314.006.
Pojoga LH, Williams JS, Yao TM, Kumar A, Raffetto JD, do Nascimento GR, et al. Histone demethylase LSD1 deficiency during high-salt diet is associated with enhanced vascular contraction, altered NO-cGMP relaxation pathway, and hypertension. Am J Physiol Heart Circ Physiol. 2011;301(5):H1862–71. doi:10.1152/ajpheart.00513.2011.
Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15(2):261–71. doi:10.1016/j.devcel.2008.07.002.
Menghini R, Casagrande V, Cardellini M, Martelli E, Terrinoni A, Amati F, et al. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009;120(15):1524–32. doi:10.1161/CIRCULATIONAHA.109.864629.
Sabatel C, Malvaux L, Bovy N, Deroanne C, Lambert V, Gonzalez ML, et al. MicroRNA-21 exhibits antiangiogenic function by targeting RhoB expression in endothelial cells. PLoS One. 2011;6(2):e16979. doi:10.1371/journal.pone.0016979.
Sequeira-Lopez ML, Weatherford ET, Borges GR, Monteagudo MC, Pentz ES, Harfe BD, et al. The microRNA-processing enzyme dicer maintains juxtaglomerular cells. J Am Soc Nephrol. 2010;21(3):460–7. doi:10.1681/ASN.2009090964.
Li D, Yang P, Xiong Q, Song X, Yang X, Liu L, et al. MicroRNA-125a/b-5p inhibits endothelin-1 expression in vascular endothelial cells. J Hypertens. 2010;28(8):1646–54. doi:10.1097/HJH.0b013e32833a4922.
Pankratz F, Bemtgen X, Zeiser R, Leonhardt F, Kreuzaler S, Hilgendorf I, et al. MicroRNA-155 exerts cell-specific antiangiogenic but proarteriogenic effects during adaptive neovascularization. Circulation. 2015;131(18):1575–89. doi:10.1161/CIRCULATIONAHA.114.014579.
Liao YC, Wang YS, Guo YC, Lin WL, Chang MH, Juo SH. Let-7g improves multiple endothelial functions through targeting transforming growth factor-beta and SIRT-1 signaling. J Am Coll Cardiol. 2014;63(16):1685–94. doi:10.1016/j.jacc.2013.09.069.
Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, et al. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23(18):2166–78. doi:10.1101/gad.1842409.
Head GA, Gueguen C, Marques FZ, Jackson KL, Eikelis N, Stevenson ER, et al. 6b.01: effect of renal denervation on blood pressure and microRNA 181a in hypertensive Schlager mice. J Hypertens. 2015;33(Suppl 1):e76. doi:10.1097/01.hjh.0000467556.72842.0d.
Marques FZ, Booth SA, Charchar FJ. The emerging role of non-coding RNA in essential hypertension and blood pressure regulation. J Hum Hypertens. 2015;29(8):459–67. doi:10.1038/jhh.2014.99.
Liu Y, Taylor NE, Lu L, Usa K, Cowley Jr AW, Ferreri NR, et al. Renal medullary microRNAs in Dahl salt-sensitive rats: miR-29b regulates several collagens and related genes. Hypertension. 2010;55(4):974–82. doi:10.1161/HYPERTENSIONAHA.109.144428.
Gu Q, Wang B, Zhang XF, Ma YP, Liu JD, Wang XZ. Contribution of renin-angiotensin system to exercise-induced attenuation of aortic remodeling and improvement of endothelial function in spontaneously hypertensive rats. Cardiovasc Pathol. 2014;23(5):298–305. doi:10.1016/j.carpath.2014.05.006.
Haugen AC, Schug TT, Collman G, Heindel JJ. Evolution of DOHaD: the impact of environmental health sciences. J Dev Orig Health Dis. 2015;6(2):55–64. doi:10.1017/S2040174414000580.
•• Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1(8489):1077–81. Undernutrition in early life increases metabolic disorder as well as hypertension.
Hoy WE, Hughson MD, Bertram JF, Douglas-Denton R, Amann K. Nephron number, hypertension, renal disease, and renal failure. J Am Soc Nephrol. 2005;16(9):2557–64. doi:10.1681/ASN.2005020172.
Paixao AD, Alexander BT. How the kidney is impacted by the perinatal maternal environment to develop hypertension. Biol Reprod. 2013;89(6):144. doi:10.1095/biolreprod.113.111823.
Hogg K, Blair JD, McFadden DE, von Dadelszen P, Robinson WP. Early onset pre-eclampsia is associated with altered DNA methylation of cortisol-signalling and steroidogenic genes in the placenta. PLoS One. 2013;8(5):e62969. doi:10.1371/journal.pone.0062969.
Drake AJ, McPherson RC, Godfrey KM, Cooper C, Lillycrop KA, Hanson MA, et al. An unbalanced maternal diet in pregnancy associates with offspring epigenetic changes in genes controlling glucocorticoid action and foetal growth. Clin Endocrinol. 2012;77(6):808–15. doi:10.1111/j.1365-2265.2012.04453.x.
Pham TD, MacLennan NK, Chiu CT, Laksana GS, Hsu JL, Lane RH. Uteroplacental insufficiency increases apoptosis and alters p53 gene methylation in the full-term IUGR rat kidney. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R962–70. doi:10.1152/ajpregu.00201.2003.
DuPont JJ, McCurley A, Davel AP, McCarthy J, Bender SB, Hong K, et al. Vascular mineralocorticoid receptor regulates microRNA-155 to promote vasoconstriction and rising blood pressure with aging. JCI Insight. 2016;1(14):e88942. doi:10.1172/jci.insight.88942.
Parthenakis F, Marketou M, Kontaraki J, Patrianakos A, Nakou H, Touloupaki M, et al. Low levels of microRNA-21 are a marker of reduced arterial stiffness in well-controlled hypertension. J Clin Hypertens (Greenwich). 2016; doi:10.1111/jch.12900.
Li H, Zhang X, Wang F, Zhou L, Yin Z, Fan J, et al. MicroRNA-21 lowers blood pressure in spontaneous hypertensive rats by upregulating mitochondrial translation. Circulation. 2016;134(10):734–51. doi:10.1161/CIRCULATIONAHA.116.023926.
Rothman AM, Arnold ND, Pickworth JA, Iremonger J, Ciuclan L, Allen RM, et al. MicroRNA-140-5p and SMURF1 regulate pulmonary arterial hypertension. J Clin Invest. 2016;126(7):2495–508. doi:10.1172/JCI83361.
Gubrij IB, Pangle AK, Pang L, Johnson LG. Reversal of microRNA dysregulation in an animal model of pulmonary hypertension. PLoS One. 2016;11(1):e0147827. doi:10.1371/journal.pone.0147827.
Acknowledgments
The current work was supported by the J.A. deSève Research Chair in Nutrition.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Hypertension and Metabolic Syndrome
Rights and permissions
About this article
Cite this article
Levy, E., Spahis, S., Bigras, JL. et al. The Epigenetic Machinery in Vascular Dysfunction and Hypertension. Curr Hypertens Rep 19, 52 (2017). https://doi.org/10.1007/s11906-017-0745-y
Published:
DOI: https://doi.org/10.1007/s11906-017-0745-y