Abstract
Preeclampsia is new onset (or worsening of preexisting) hypertension that occurs during pregnancy. It is accompanied by chronic inflammation, intrauterine growth restriction, elevated anti-angiogenic factors, and can occur with or without proteinuria. Although the exact etiology is unknown, it is thought that preeclampsia begins early in gestation with reduced uterine spiral artery remodeling leading to decreased vasculogenesis of the placenta as the pregnancy progresses. Soluble factors, stimulated by the ischemic placenta, shower the maternal vascular endothelium and are thought to cause endothelial dysfunction and to contribute to the development of hypertension during pregnancy. Due to the difficulty in studying such soluble factors in pregnant women, various animal models have been designed. Studies from these models have contributed to a better understanding of how factors released in response to placental ischemia may lead to increased blood pressure and reduced fetal weight during pregnancy. This review will highlight various animal models and the major findings indicating the importance of placental ischemia to lead to the pathophysiology observed in preeclamptic patients.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Keyes LE et al. Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia. Pediatr Res. 2003;54(1):20–5.
Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123(24):2856–69.
Thadhani RI, Johnson RJ, Karumanchi SA. Hypertension during pregnancy: a disorder begging for pathophysiological support. Hypertension. 2005;46(6):1250–1.
Redman CW. Preeclampsia: a multi-stress disorder. Rev Med Interne. 2011;32 Suppl 1:S41–4.
Redman CW, Sargent L. Immunology of pre-eclampsia. Am J Reprod Immunol. 2010;63(6):534–43. Reviews the importance of immune cells and inflammation during normal pregnancy and preeclampsia, respectively
Brown CE et al. Low-dose aspirin. II. Relationship of angiotensin II pressor responses, circulating eicosanoids, and pregnancy outcome. Am J Obstet Gynecol. 1990;163(6 Pt 1):1853–61.
Hladunewich M, Karumanchi SA, Lafayette R. Pathophysiology of the clinical manifestations of preeclampsia. Clin J Am Soc Nephrol. 2007;2(3):543–9.
Khalil RA, Granger JP. Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. Am J Physiol Regul Integr Comp Physiol. 2002;283(1):R29–45.
Lunell NO et al. Uteroplacental blood flow in pregnancy induced hypertension. Scand J Clin Lab Investig Suppl. 1984;169:28–35.
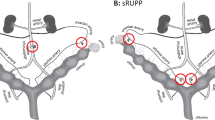
Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am J Physiol Heart Circ Physiol. 2012;303(1):H1–8.
Gilbert JS et al. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294(2):H541–50.
Odegard RA et al. Preeclampsia and fetal growth. Obstet Gynecol. 2000;96(6):950–5.
Roberts JM, Escudero C. The placenta in preeclampsia. Hypertens Pregnancy. 2012;2(2):72–83.
Ahmed A et al. Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen--a review. Placenta. 2000;21 Suppl A:S16–24.
Rodesch F et al. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80(2):283–5.
Zhou Y et al. Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J Clin Invest. 1993;91(3):950–60.
Zhou Y et al. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest. 1997;99(9):2139–51.
Mabie WC et al. A longitudinal study of cardiac output in normal human pregnancy. Am J Obstet Gynecol. 1994;170(3):849–56.
Jauniaux E, Burton GJ. Morphological and biological effects of maternal exposure to tobacco smoke on the feto-placental unit. Early Hum Dev. 2007;83(11):699–706.
Jauniaux E et al. Amniotic gas values and acid–base status during acute maternal hyperoxemia and hypoxemia in the early fetal sheep. Am J Obstet Gynecol. 2000;182(3):661–5.
Jauniaux E et al. In-vivo measurement of intrauterine gases and acid–base values early in human pregnancy. Hum Reprod. 1999;14(11):2901–4.
Lala PK, Chakraborty C. Factors regulating trophoblast migration and invasiveness: possible derangements contributing to pre-eclampsia and fetal injury. Placenta. 2003;24(6):575–87.
Wells M. The pathology of gestational trophoblastic disease: recent advances. Pathology. 2007;39(1):88–96.
King A. Uterine leukocytes and decidualization. Hum Reprod Update. 2000;6(1):28–36.
Trundley A, Moffett A. Human uterine leukocytes and pregnancy. Tissue Antigens. 2004;63(1):1–12.
Williams PJ et al. Decidual leucocyte populations in early to late gestation normal human pregnancy. J Reprod Immunol. 2009;82(1):24–31. Describes early immune pregnancy cell populations.
Craven CM, Morgan T, Ward K. Decidual spiral artery remodelling begins before cellular interaction with cytotrophoblasts. Placenta. 1998;19(4):241–52.
Hanna J et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12(9):1065–74.
Somerset DA et al. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112(1):38–43.
Tilburgs T et al. Evidence for a selective migration of fetus-specific CD4 + CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J Immunol. 2008;180(8):5737–45. Describes specific methods for T cells stainings and populations in pregnancy.
Veenstra van Nieuwenhoven AL, Heineman MJ, Faas MM, The immunology of successful pregnancy. Hum Reprod Update. 2003;9(4):347–57.
Freeman DJ et al. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension. 2004;44(5):708–14.
LaMarca B, Cornelius D, Wallace K. Elucidating immune mechanisms causing hypertension during pregnancy. Physiology (Bethesda). 2013;28(4):225–33.
Cornelius DC et al. Administration of interleukin-17 soluble receptor C suppresses TH17 cells, oxidative stress, and hypertension in response to placental ischemia during pregnancy. Hypertension. 2013;62(6):1068–73.
Irani RA et al. Autoantibody-mediated angiotensin receptor activation contributes to preeclampsia through tumor necrosis factor-alpha signaling. Hypertension. 2010;55(5):1246–53.
Prins JR et al. Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertens Pregnancy. 2009;28(3):300–11.
Santner-Nanan B et al. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009;183(11):7023–30. First description of changes in T regs and TH17 during PE.
Wallace K et al. CD4+ T-helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension. 2011;57(5):949–55.
Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37(3):240–9.
Dhillion P et al. IL-17-mediated oxidative stress is an important stimulator of AT1-AA and hypertension during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2012;303(4):R353–8.
Granger JP et al. Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Hypertension. 2001;38(3 Pt 2):718–22.
Granger JP et al. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation. 2002;9(3):147–60.
LaMarca BB et al. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension. 2005;46(1):82–6.
LaMarca BD et al. Pathophysiology of hypertension in response to placental ischemia during pregnancy: a central role for endothelin? Gend Med. 2008;5 Suppl A:S133–8.
Herse F, LaMarca B. Angiotensin II type 1 receptor autoantibody (AT1-AA)-mediated pregnancy hypertension. Am J Reprod Immunol. 2013;69(4):413–8.
LaMarca B, Wallace K, Granger J. Role of angiotensin II type I receptor agonistic autoantibodies (AT1-AA) in preeclampsia. Curr Opin Pharmacol. 2011;11(2):175–9.
Parrish MR et al. Angiotensin II type 1 autoantibody induced hypertension during pregnancy is associated with renal endothelial dysfunction. Gend Med. 2011;8(3):184–8.
Wenzel K et al. Angiotensin II type 1 receptor antibodies and increased angiotensin II sensitivity in pregnant rats. Hypertension. 2011;58(1):77–84.
Zhou CC et al. Autoantibody from women with preeclampsia induces soluble Fms-like tyrosine kinase-1 production via angiotensin type 1 receptor and calcineurin/nuclear factor of activated T-cells signaling. Hypertension. 2008;51(4):1010–9.
Podjarny E, Losonczy G, Baylis C. Animal models of preeclampsia. Semin Nephrol. 2004;24(6):596–606.
Abitbol MM. A simplified technique to produce toxemia in the pregnant dog. Am J Obstet Gynecol. 1981;139(5):526–34.
Woods LL, Brooks VL. Role of the renin-angiotensin system in hypertension during reduced uteroplacental perfusion pressure. Am J Physiol. 1989;257(1 Pt 2):R204–9.
Abitbol MM, Driscoll SG, Ober WB. Placental lesions in experimental toxemia in the rabbit. Am J Obstet Gynecol. 1976;125(7):942–8.
Abitbol MM et al. Production of experimental toxemia in the pregnant rabbit. Am J Obstet Gynecol. 1976;124(5):460–70.
Combs CA et al. Experimental preeclampsia produced by chronic constriction of the lower aorta: validation with longitudinal blood pressure measurements in conscious rhesus monkeys. Am J Obstet Gynecol. 1993;169(1):215–23.
Zhou Y et al. Increased depth of trophoblast invasion after chronic constriction of the lower aorta in rhesus monkeys. Am J Obstet Gynecol. 1993;169(1):224–9.
Cavanagh D et al. Pregnancy-induced hypertension: development of a model in the pregnant primate (Papio anubis). Am J Obstet Gynecol. 1985;151(7):987–99.
Crews JK et al. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension. 2000;35(1 Pt 2):367–72.
Alexander BT et al. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37(4):1191–5.
Murphy JG et al. Enhanced [Ca2+]i in renal arterial smooth muscle cells of pregnant rats with reduced uterine perfusion pressure. Am J Physiol Heart Circ Physiol. 2003;284(1):H393–403.
Roberts L et al. Enhanced endothelin synthesis by endothelial cells exposed to sera from pregnant rats with decreased uterine perfusion. Hypertension. 2006;47(3):615–8.
Alexander BT et al. Tumor necrosis factor-alpha-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am J Hypertens. 2002;15(2 Pt 1):170–5.
Wallukat G et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103(7):945–52. First description of AT1-AA during PE.
Sedeek M et al. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am J Hypertens. 2008;21(10):1152–6.
Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007;50(6):1142–7.
Gilbert JS et al. Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression. Hypertension. 2009;53(2):399–403.
Kupferminc MJ et al. Tumor necrosis factor-alpha is elevated in plasma and amniotic fluid of patients with severe preeclampsia. Am J Obstet Gynecol. 1994;170(6):1752–7. discussion 1757–9.
Gadonski G et al. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of interleukin 6. Hypertension. 2006;48(4):711–6.
Heikkinen J et al. Phenotypic characterization of regulatory T cells in the human decidua. Clin Exp Immunol. 2004;136(2):373–8.
Saito S et al. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63(6):601–10.
Sakaguchi S et al. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–87.
Sasaki Y et al. Decidual and peripheral blood CD4 + CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10(5):347–53.
Lash GE, Robson SC, Bulmer JN. Review: functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta. 2010;31(Suppl):S87–92.
Munoz-Suano A, Hamilton AB, Betz AG. Gimme shelter: the immune system during pregnancy. Immunol Rev. 2011;241(1):20–38.
Tilburgs T et al. Expression of NK cell receptors on decidual T cells in human pregnancy. J Reprod Immunol. 2009;80(1–2):22–32.
Zenclussen AC et al. Introducing a mouse model for pre-eclampsia: adoptive transfer of activated Th1 cells leads to pre-eclampsia-like symptoms exclusively in pregnant mice. Eur J Immunol. 2004;34(2):377–87.
Novotny SR et al. Activating autoantibodies to the angiotensin II type I receptor play an important role in mediating hypertension in response to adoptive transfer of CD4+ T lymphocytes from placental ischemic rats. Am J Physiol Regul Integr Comp Physiol. 2012;302(10):R1197–201.
Novotny S, et al. CD4 T cells play a critical role in mediating hypertension in response to placental ischemia. J Hypertens (Los Angel). 2013;2.
Cornelius DC, et al. Administration of interleukin-17 soluble receptor C suppresses TH17 cells, oxidative stress, and hypertension in response to placental ischemia during pregnancy. Hypertension. 2013.
Kleinewietfeld M, Hafler DA. Regulatory T cells in autoimmune neuroinflammation. Immunol Rev. 2014;259(1):231–44.
Quinn KH et al. The unique pathophysiology of early-onset severe preeclampsia: role of decidual T regulatory cells. J Reprod Immunol. 2011;91(1–2):76–82.
Sasaki Y et al. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia. Clin Exp Immunol. 2007;149(1):139–45.
von Rango U. Fetal tolerance in human pregnancy--a crucial balance between acceptance and limitation of trophoblast invasion. Immunol Lett. 2008;115(1):21–32.
Zenclussen AC et al. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4 + CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005;166(3):811–22.
Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5(3):266–71.
Redman CW et al. Review: does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta. 2012;33(Suppl):S48–54.
Chamley LW et al. Trophoblast deportation: just a waste disposal system or antigen sharing? J Reprod Immunol. 2011;88(2):99–105.
Lau SY et al. Necrotic trophoblast debris increases blood pressure during pregnancy. J Reprod Immunol. 2013;97(2):175–82.
Hubel CA et al. Agonistic angiotensin II type 1 receptor autoantibodies in postpartum women with a history of preeclampsia. Hypertension. 2007;49(3):612–7.
Brewer J et al. Endothelin-1, oxidative stress, and endogenous angiotensin II: mechanisms of angiotensin II type I receptor autoantibody-enhanced renal and blood pressure response during pregnancy. Hypertension. 2013;62(5):886–92.
LaMarca B et al. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension. 2009;54(4):905–9.
Parrish MR et al. The effect of immune factors, tumor necrosis factor-alpha, and agonistic autoantibodies to the angiotensin II type I receptor on soluble fms-like tyrosine-1 and soluble endoglin production in response to hypertension during pregnancy. Am J Hypertens. 2010;23(8):911–6.
Parrish MR et al. Hypertension in response to AT1-AA: role of reactive oxygen species in pregnancy-induced hypertension. Am J Hypertens. 2011;24(7):835–40.
Xia Y, Kellems RE. Angiotensin receptor agonistic autoantibodies and hypertension: preeclampsia and beyond. Circ Res. 2013;113(1):78–87.
Quitterer U, Lother H, Abdalla S. AT1 receptor heterodimers and angiotensin II responsiveness in preeclampsia. Semin Nephrol. 2004;24(2):115–9.
AbdAlla S et al. Increased AT(1) receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness. Nat Med. 2001;7(9):1003–9.
Nagamatsu T et al. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145(11):4838–45.
Nevo O et al. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R1085–93.
Makris A et al. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int. 2007;71(10):977–84.
Maynard SE et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–58.
Bridges JP et al. Oxidative stress contributes to soluble fms-like tyrosine kinase-1 induced vascular dysfunction in pregnant rats. Am J Hypertens. 2009;22(5):564–8.
Murphy SR et al. Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension. 2010;55(2):394–8.
Li Z et al. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension. 2007;50(4):686–92.
Bergmann A et al. Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J Cell Mol Med. 2010;14(6B):1857–67.
Murphy S et al. Role of endothelin in mediating soluble fms-like tyrosine kinase 1 induced hypertension in pregnant rats. Hypertension. 2010;55:394–8.
Gilbert JS et al. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placental ischemia-induced hypertension. Hypertension. 2010;55(2):380–5.
Davisson RL et al. Discovery of a spontaneous genetic mouse model of preeclampsia. Hypertension. 2002;39(2 Pt 2):337–42.
Wallace K et al. Hypertension in response to CD4(+) T cells from reduced uterine perfusion pregnant rats is associated with activation of the endothelin-1 system. Am J Physiol Regul Integr Comp Physiol. 2012;303(2):R144–9.
Woods AK et al. Adenoviral delivery of VEGF121 early in pregnancy prevents spontaneous development of preeclampsia in BPH/5 mice. Hypertension. 2011;57(1):94–102.
Shah DM. Role of the renin-angiotensin system in the pathogenesis of preeclampsia. Am J Physiol Ren Physiol. 2005;288(4):F614–25.
Takimoto E et al. Hypertension induced in pregnant mice by placental renin and maternal angiotensinogen. Science. 1996;274(5289):995–8.
Bohlender J, Ganten D, Luft FC. Rats transgenic for human renin and human angiotensinogen as a model for gestational hypertension. J Am Soc Nephrol. 2000;11(11):2056–61.
Verlohren S et al. Uterine vascular function in a transgenic preeclampsia rat model. Hypertension. 2008;51(2):547–53.
Falcao S et al. Mice overexpressing both human angiotensinogen and human renin as a model of superimposed preeclampsia on chronic hypertension. Hypertension. 2009;54(6):1401–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Drs. LaMarca, Amaral, Harmon, Cornelius, Jessica, and Cunningham declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Preeclampsia
Rights and permissions
About this article
Cite this article
LaMarca, B., Amaral, L.M., Harmon, A.C. et al. Placental Ischemia and Resultant Phenotype in Animal Models of Preeclampsia. Curr Hypertens Rep 18, 38 (2016). https://doi.org/10.1007/s11906-016-0633-x
Published:
DOI: https://doi.org/10.1007/s11906-016-0633-x