Abstract
The impaired capacity of the kidney to excrete sodium plays an essential role in the development of hypertension. Adrenal corticosteroids control renal handling of sodium by regulating tubular sodium reabsorption in the distal nephron where both mineralocorticoid receptors (MR) and glucocorticoid receptors are expressed. In addition, cell type- and segment-specific expression of 11β-HSD2 and sodium transporters such as Na–Cl cotransporter (NCC), epithelial sodium channel (ENaC), and pendrin/Na+-driven Cl−/HCO3 − exchanger (NDCBE) builds a distinctive model of sodium transport in the aldosterone-sensitive distal nephron. Aberrant MR activation in the distal nephron triggers salt-sensitive hypertension and hypokalemia through inappropriate sodium reabsorption and potassium secretion. However, MR activity is not necessarily modulated by the ligand alone. Recently, several lines of evidence revealed alternative mechanisms that regulate the activity of MR in a ligand-independent manner or through ligand binding modulation. This review summarizes the disorders related to MR activation in individual tubular cells and highlights the renal mechanism of salt-sensitive hypertension and new approaches for the prevention and treatment of this disease.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lawes CM, Vander Hoorn S, Rodgers A, et al. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–8.
Gu Q, Burt VL, Dillon CF, et al. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health and Nutrition Examination Survey, 2001 to 2010. Circulation. 2012;126:2105–14.
Luft FC, Weinberger MH. Heterogeneous responses to changes in dietary salt intake: the salt-sensitivity paradigm. Am J Clin Nutr. 1997;65:612S–7.
Fujita T, Henry WL, Bartter FC, et al. Factors influencing blood pressure in salt-sensitive patients with hypertension. Am J Med. 1980;69:334–44.
Guyton AC. The surprising kidney-fluid mechanism for pressure control—its infinite gain! Hypertension. 1990;16:725–30.
Hall JE, Mizelle HL, Hildebrandt DA, et al. Abnormal pressure natriuresis. A cause or a consequence of hypertension? Hypertension. 1990;15:547–59.
Coffman TM. Under pressure: the search for the essential mechanisms of hypertension. Nat Med. 2011;17:1402–9.
Reilly RF, Ellison DH. Mammalian distal tubule: physiology, pathophysiology, and molecular anatomy. Physiol Rev. 2000;80:277–313.
Rossier BC, Staub OHummler E. Genetic dissection of sodium and potassium transport along the aldosterone-sensitive distal nephron: importance in the control of blood pressure and hypertension. FEBS Lett. 2013;587:1929–41.
Albiston AL, Obeyesekere VR, Smith RE, et al. Cloning and tissue distribution of the human 11 beta-hydroxysteroid dehydrogenase type 2 enzyme. Mol Cell Endocrinol. 1994;105:R11–7.
Kyossev Z, Walker PD Reeves WB. Immunolocalization of NAD-dependent 11 beta-hydroxysteroid dehydrogenase in human kidney and colon. Kidney Int. 1996;49:271–81.
Campean V, Kricke J, Ellison D, et al. Localization of thiazide-sensitive Na(+)-Cl(-) cotransport and associated gene products in mouse DCT. Am J Physiol Renal Physiol. 2001;281:F1028–35.
Rozansky DJ, Cornwall T, Subramanya AR, et al. Aldosterone mediates activation of the thiazide-sensitive Na-Cl cotransporter through an SGK1 and WNK4 signaling pathway. J Clin Invest. 2009;119:2601–12.
Faresse N, Lagnaz D, Debonneville A, et al. Inducible kidney-specific Sgk1 knockout mice show a salt-losing phenotype. Am J Physiol Renal Physiol. 2012;302:F977–85.
Shi PP, Cao XR, Sweezer EM, et al. Salt-sensitive hypertension and cardiac hypertrophy in mice deficient in the ubiquitin ligase Nedd4-2. Am J Physiol Renal Physiol. 2008;295:F462–70.
Ronzaud C, Loffing-Cueni D, Hausel P, et al. Renal tubular NEDD4-2 deficiency causes NCC-mediated salt-dependent hypertension. J Clin Invest. 2013;123:657–65.
Ko B, Mistry AC, Hanson L, et al. Aldosterone acutely stimulates NCC activity via a SPAK-mediated pathway. Am J Physiol Renal Physiol. 2013;305:F645–52.
Elvira-Matelot E, Zhou XO, Farman N, et al. Regulation of WNK1 expression by miR-192 and aldosterone. J Am Soc Nephrol. 2010;21:1724–31.
Mu S, Shimosawa T, Ogura S, et al. Epigenetic modulation of the renal beta-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nat Med. 2011;17:573–80.
Williams GH, Burgess E, Kolloch RE, et al. Efficacy of eplerenone versus enalapril as monotherapy in systemic hypertension. Am J Cardiol. 2004;93:990–6.
Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17.
Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21.
Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21.
Brilla CG, Pick R, Tan LB, et al. Remodeling of the rat right and left ventricles in experimental hypertension. Circ Res. 1990;67:1355–64.
Brilla CG, Matsubara LSWeber KT. Anti-aldosterone treatment and the prevention of myocardial fibrosis in primary and secondary hyperaldosteronism. J Mol Cell Cardiol. 1993;25:563–75.
Greene EL, Kren SHostetter TH. Role of aldosterone in the remnant kidney model in the rat. J Clin Invest. 1996;98:1063–8.
Blasi ER, Rocha R, Rudolph AE, et al. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63:1791–800.
Bochud M, Nussberger J, Bovet P, et al. Plasma aldosterone is independently associated with the metabolic syndrome. Hypertension. 2006;48:239–45.
Kidambi S, Kotchen JM, Grim CE, et al. Association of adrenal steroids with hypertension and the metabolic syndrome in blacks. Hypertension. 2007;49:704–11.
Rocchini AP, Key J, Bondie D, et al. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N Engl J Med. 1989;321:580–5.
Nagase M, Yoshida S, Shibata S, et al. Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: possible contribution of fat-derived factors. J Am Soc Nephrol. 2006;17:3438–46.
Nagase M, Matsui H, Shibata S, et al. Salt-induced nephropathy in obese spontaneously hypertensive rats via paradoxical activation of the mineralocorticoid receptor: role of oxidative stress. Hypertension. 2007;50:877–83.
de Paula RB, da Silva AAHall JE. Aldosterone antagonism attenuates obesity-induced hypertension and glomerular hyperfiltration. Hypertension. 2004;43:41–7.
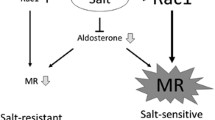
Shibata S, Mu S, Kawarazaki H, et al. PMC3148723; Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest. 2011;121:3233–43.
Farjah M, Roxas BP, Geenen DL, et al. Dietary salt regulates renal SGK1 abundance: relevance to salt sensitivity in the Dahl rat. Hypertension. 2003;41:874–8.
Aoi W, Niisato N, Sawabe Y, et al. Aldosterone-induced abnormal regulation of ENaC and SGK1 in Dahl salt-sensitive rat. Biochem Biophys Res Commun. 2006;341:376–81.
Luther JM, Luo P, Wang Z, et al. PMC3434275; Aldosterone deficiency and mineralocorticoid receptor antagonism prevent angiotensin II-induced cardiac, renal, and vascular injury. Kidney Int. 2012.
Mihailidou AS, Le Loan TY, Mardini M, et al. Glucocorticoids activate cardiac mineralocorticoid receptors during experimental myocardial infarction. Hypertension. 2009;54:1306–12.
Funder JW. Minireview: aldosterone and mineralocorticoid receptors: past, present, and future. Endocrinology. 2010;151:5098–102.
Massaad C, Houard N, Lombes M, et al. Modulation of human mineralocorticoid receptor function by protein kinase A. Mol Endocrinol. 1999;13:57–65.
Yokota K, Shibata H, Kurihara I, et al. Coactivation of the N-terminal transactivation of mineralocorticoid receptor by Ubc9. J Biol Chem. 2007;282:1998–2010.
Shibata S, Nagase M, Yoshida S, et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14:1370–6.
Pavlov TS, Levchenko VStaruschenko A. Role of Rho GDP dissociation inhibitor alpha in control of epithelial sodium channel (ENaC)-mediated sodium reabsorption. J Biol Chem. 2014;289:28651–9.
Tapia-Castillo A, Carvajal CA, Campino C, et al. Polymorphisms in the RAC1 gene are associated with hypertension risk factors in a Chilean pediatric population. Am J Hypertens. 2014;27:299–307.
Akilesh S, Suleiman H, Yu H, et al. Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J Clin Invest. 2011;121:4127–37.
Gupta IR, Baldwin C, Auguste D, et al. ARHGDIA: a novel gene implicated in nephrotic syndrome. J Med Genet. 2013;50:330–8.
Gee HY, Saisawat P, Ashraf S, et al. ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. J Clin Invest. 2013;123:3243–53.
Kawarazaki W, Nagase M, Yoshida S, et al. PMC3358757; angiotensin II- and salt-induced kidney injury through Rac1-mediated mineralocorticoid receptor activation. J Am Soc Nephrol. 2012;23:997–1007. This report demonstrated that salt-induced hypertension and renal injury in renin and angiotensinogen-overproducing transgenic mice is mediated by Rac1-mediated MR activation in the kidney.
Kobori H, Nishiyama A, Abe Y, et al. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension. 2003;41:592–7.
Kobori H, Nangaku M, Navar LG, et al. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–87.
Terada Y, Knepper MA. Thiazide-sensitive NaCl absorption in rat cortical collecting duct. Am J Physiol. 1990;259:F519–28.
Tomita K, Pisano JJ, Burg MB, et al. Effects of vasopressin and bradykinin on anion transport by the rat cortical collecting duct. Evidence for an electroneutral sodium chloride transport pathway. J Clin Invest. 1986;77:136–41.
Tomita K, Pisano JJ, Knepper MA. Control of sodium and potassium transport in the cortical collecting duct of the rat. Effects of bradykinin, vasopressin, and deoxycorticosterone. J Clin Invest. 1985;76:132–6.
Leviel F, Hubner CA, Houillier P, et al. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest. 2010;120:1627–35.
Jacques T, Picard N, Miller RL, et al. Overexpression of pendrin in intercalated cells produces chloride-sensitive hypertension. J Am Soc Nephrol. 2013;24:1104–13.
Chambrey R, Kurth I, Peti-Peterdi J, et al. Renal intercalated cells are rather energized by a proton than a sodium pump. Proc Natl Acad Sci U S A. 2013;110:7928–33.
Smith AN, Skaug J, Choate KA, et al. Mutations in ATP6N1B, encoding a new kidney vacuolar proton pump 116-kD subunit, cause recessive distal renal tubular acidosis with preserved hearing. Nat Genet. 2000;26:71–5.
Gueutin V, Vallet M, Jayat M, et al. Renal beta-intercalated cells maintain body fluid and electrolyte balance. J Clin Invest. 2013;123:4219–31.
Sebastian A, McSherry EMorris Jr RC. Renal potassium wasting in renal tubular acidosis (RTA): its occurrence in types 1 and 2 RTA despite sustained correction of systemic acidosis. J Clin Invest. 1971;50:667–78.
Sebastian A, McSherry EMorris Jr RC. Impaired renal conservation of sodium and chloride during sustained correction of systemic acidosis in patients with type 1, classic renal tubular acidosis. J Clin Invest. 1976;58:454–69.
Frische S, Kwon TH, Frokiaer J, et al. Regulated expression of pendrin in rat kidney in response to chronic NH4Cl or NaHCO3 loading. Am J Physiol Renal Physiol. 2003;284:F584–93.
Pech V, Pham TD, Hong S, et al. Pendrin modulates ENaC function by changing luminal HCO3-. J Am Soc Nephrol. 2010;21:1928–41.
Kurtz TW, Al-Bander HA, Morris Jr RC. “Salt-sensitive” essential hypertension in men. Is the sodium ion alone important? N Engl J Med. 1987;317:1043–8.
Kurtz TW, Morris Jr RC. Dietary chloride as a determinant of “sodium-dependent” hypertension. Science. 1983;222:1139–41.
Schmidlin O, Tanaka M, Bollen AW, et al. Chloride-dominant salt sensitivity in the stroke-prone spontaneously hypertensive rat. Hypertension. 2005;45:867–73.
Tanaka M, Schmidlin O, Yi SL, et al. Genetically determined chloride-sensitive hypertension and stroke. Proc Natl Acad Sci U S A. 1997;94:14748–52.
Whitescarver SA, Ott CE, Jackson BA, et al. Salt-sensitive hypertension: contribution of chloride. Science. 1984;223:1430–2.
Ponce-Coria J, San-Cristobal P, Kahle KT, et al. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci U S A. 2008;105:8458–63.
Pacheco-Alvarez D, Gamba G. WNK3 is a putative chloride-sensing kinase. Cell Physiol Biochem. 2011;28:1123–34.
Bazua-Valenti S, Chavez-Canales M, Rojas-Vega L, et al. The effect of WNK4 on the Na+-Cl- cotransporter is modulated by intracellular chloride. J Am Soc Nephrol. 2014.
Piala AT, Moon TM, Akella R, et al. Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal. 2014;7:ra41.
San-Cristobal P, Pacheco-Alvarez D, Richardson C, et al. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci U S A. 2009;106:4384–9.
Sato Y, Ogata E, Fujita T. Role of chloride in angiotensin II-induced salt-sensitive hypertension. Hypertension. 1991;18:622–9.
O’Neil RG, Helman SI. Transport characteristics of renal collecting tubules: influences of DOCA and diet. Am J Physiol. 1977;233:F544–58.
Stoner LC, Burg MB, Orloff J. Ion transport in cortical collecting tubule; effect of amiloride. Am J Physiol. 1974;227:453–9.
Wall SM, Kim YH, Stanley L, et al. NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: role in Cl- conservation. Hypertension. 2004;44:982–7.
Pech V, Kim YH, Weinstein AM, et al. Angiotensin II increases chloride absorption in the cortical collecting duct in mice through a pendrin-dependent mechanism. Am J Physiol Renal Physiol. 2007;292:F914–20.
Azroyan A, Morla L, Crambert G, et al. Regulation of pendrin by cAMP: possible involvement in beta-adrenergic-dependent NaCl retention. Am J Physiol Renal Physiol. 2012;302:F1180–7.
Verlander JW, Hassell KA, Royaux IE, et al. Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: role of pendrin in mineralocorticoid-induced hypertension. Hypertension. 2003;42:356–62.
Pelzl L, Pakladok T, Pathare G, et al. DOCA sensitive pendrin expression in kidney, heart, lung and thyroid tissues. Cell Physiol Biochem. 2012;30:1491–501.
Shibata S, Rinehart J, Zhang J, et al. Mineralocorticoid receptor phosphorylation regulates ligand binding and renal response to volume depletion and hyperkalemia. Cell Metab. 2013;18:660–71. This study revealed the unique mechanism by which renal intercalated cells regulate the activity of MR through phosphorylation, which switches NaCl transport of them and leads to distinct adaptive responses to volume depletion and hyperkalemia.
Eladari D, Chambrey R, Peti-Peterdi J. A new look at electrolyte transport in the distal tubule. Annu Rev Physiol. 2012;74:325–49.
Arroyo JP, Ronzaud C, Lagnaz D, et al. Aldosterone paradox: differential regulation of ion transport in distal nephron. Physiology (Bethesda). 2011;26:115–23.
Ando K, Ohtsu H, Uchida S, et al. Anti-albuminuric effect of the aldosterone blocker eplerenone in non-diabetic hypertensive patients with albuminuria: a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2:944-953. This double-blind, randomized, placebo-controlled trial showed that the addition of the selective MR blocker eplerenone to renin–angiotensin system inhibitor therapy significantly reduces albuminuria in hypertensive patients with non-diabetic chronic kidney disease.
Acknowledgments
This work was supported by Japan Society for the Promotion of Science KAKENHI (Grant Number 21229012).
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Nobuhiro Ayuzawa and Toshiro Fujita declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Hypertension and the Kidney
Rights and permissions
About this article
Cite this article
Ayuzawa, N., Fujita, T. Activation of Mineralocorticoid Receptor in Salt-Sensitive Hypertension. Curr Hypertens Rep 17, 44 (2015). https://doi.org/10.1007/s11906-015-0552-2
Published:
DOI: https://doi.org/10.1007/s11906-015-0552-2