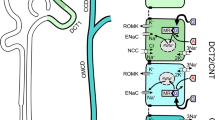
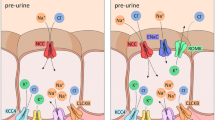
Abstract
The kidney continuously adapts daily renal excretion of NaCl to match dietary intakes in order to maintain the NaCl content of the body, and keep vascular volume constant. Any situation that leads to NaCl retention favors a rise in blood pressure. The aldosterone-sensitive distal nephron, which contains two main types of cells, principal (PC) and intercalated (IC) cells, is an important site for the final regulation of urinary Na+ excretion. Research over the past 20 years established a paradigm in which PCs are the exclusive site of Na+ absorption while ICs are solely dedicated to acid-base transport. Recent studies have revealed the unexpected importance of ICs for NaCl reabsorption. Here, we review the mechanisms of Na+ and Cl− transport in the aldosterone-sensitive distal nephron, with emphasis on the role of ICs in maintaining NaCl balance and normal blood pressure.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41(6):677–87.
Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41(6):666–76.
Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104(4):545–56.
Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288(23):2981–97.
Hansson JH, Nelson-Williams C, Suzuki H, Schild L, Shimkets R, Lu Y, et al. Hypertension caused by a truncated epithelial sodium channel gamma subunit: genetic heterogeneity of Liddle syndrome. Nat Genet. 1995;11(1):76–82.
Shimkets RA, Warnock DG, Bositis CM, Nelson-Williams C, Hansson JH, Schambelan M, et al. Liddle’s syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell. 1994;79(3):407–14.
Hansson JH, Schild L, Lu Y, Wilson TA, Gautschi I, Shimkets R, et al. A de novo missense mutation of the beta subunit of the epithelial sodium channel causes hypertension and Liddle syndrome, identifying a proline-rich segment critical for regulation of channel activity. Proc Natl Acad Sci U S A. 1995;92(25):11495–9.
Liddle D, Foss BM. A vertical-horizontal illusion for movement perceived tactually. Nature. 1963;197:108.
Scheinman SJ, Guay-Woodford LM, Thakker RV, Warnock DG. Genetic disorders of renal electrolyte transport. N Engl J Med. 1999;340(15):1177–87.
Rotin D, Staub O. Nedd4-2 and the regulation of epithelial sodium transport. Front Physiol. 2012;3:212.
Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, et al. Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na(+) channel cell surface expression. EMBO J. 2001;20(24):7052–9.
Shi PP, Cao XR, Sweezer EM, Kinney TS, Williams NR, Husted RF, et al. Salt-sensitive hypertension and cardiac hypertrophy in mice deficient in the ubiquitin ligase Nedd4-2. Am J Physiol Ren Physiol. 2008;295(2):F462–70.
Ronzaud C, Loffing-Cueni D, Hausel P, Debonneville A, Malsure SR, Fowler-Jaeger N, et al. Renal tubular NEDD4-2 deficiency causes NCC-mediated salt-dependent hypertension. J Clin Invest. 2013;123(2):657–65.
Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293(5532):1107–12.
Heise CJ, Xu BE, Deaton SL, Cha SK, Cheng CJ, Earnest S, et al. Serum and glucocorticoid-induced kinase (SGK) 1 and the epithelial sodium channel are regulated by multiple with no lysine (WNK) family members. J Biol Chem. 2010;285(33):25161–7.
Xu BE, Stippec S, Chu PY, Lazrak A, Li XJ, Lee BH, et al. WNK1 activates SGK1 to regulate the epithelial sodium channel. Proc Natl Acad Sci U S A. 2005;102(29):10315–20.
Ring AM, Cheng SX, Leng Q, Kahle KT, Rinehart J, Lalioti MD, et al. WNK4 regulates activity of the epithelial Na + channel in vitro and in vivo. Proc Natl Acad Sci U S A. 2007;104(10):4020–4.
Shibata S, Zhang J, Puthumana J, Stone KL, Lifton RP. Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4. Proc Natl Acad Sci U S A. 2013;110(19):7838–43.
Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, et al. Molecular pathogenesis of pseudohypoaldosteronism type II: generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab. 2007;5(5):331–44.
Farfel Z, Mayan H, Yaacov Y, Mouallem M, Shaharabany M, Pauzner R, et al. WNK4 regulates airway Na + transport: study of familial hyperkalaemia and hypertension. Eur J Clin Investig. 2005;35(6):410–5.
Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13(5):1131–5.
Prieto-Carrasquero MC, Botros FT, Kobori H, Navar LG. Collecting duct renin: a major player in angiotensin II-dependent hypertension. J Am Soc Hypertens. 2009;3(2):96–104.
Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, et al. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34(6):1265–74.
Komlosi P, Fuson AL, Fintha A, Peti-Peterdi J, Rosivall L, Warnock DG, et al. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension. 2003;42(2):195–9.
Gonzalez-Villalobos RA, Billet S, Kim C, Satou R, Fuchs S, Bernstein KE, et al. Intrarenal angiotensin-converting enzyme induces hypertension in response to angiotensin I infusion. J Am Soc Nephrol. 2011;22(3):449–59.
Kobori H, Ozawa Y, Satou R, Katsurada A, Miyata K, Ohashi N, et al. Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Ren Physiol. 2007;293(3):F938–45.
Sachetelli S, Liu Q, Zhang SL, Liu F, Hsieh TJ, Brezniceanu ML, et al. RAS blockade decreases blood pressure and proteinuria in transgenic mice overexpressing rat angiotensinogen gene in the kidney. Kidney Int. 2006;69(6):1016–23.
Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, Giani JF, Nguyen MT, Riquier-Brison AD, et al. The absence of intrarenal ACE protects against hypertension. J Clin Invest. 2013;123(5):2011–23. This pionering study shows that intrarenal renin and angiotensin II regulate blood pressure independently from their systemic activity.
Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem. 2009;284(31):20447–51.
Planes C, Randrianarison NH, Charles RP, Frateschi S, Cluzeaud F, Vuagniaux G, et al. ENaC-mediated alveolar fluid clearance and lung fluid balance depend on the channel-activating protease 1. EMBO Mol Med. 2010;2(1):26–37.
Narikiyo T, Kitamura K, Adachi M, Miyoshi T, Iwashita K, Shiraishi N, et al. Regulation of prostasin by aldosterone in the kidney. J Clin Invest. 2002;109(3):401–8.
Picard N, Eladari D, El Moghrabi S, Planes C, Bourgeois S, Houillier P, et al. Defective ENaC processing and function in tissue kallikrein-deficient mice. J Biol Chem. 2008;283(8):4602–11.
Patel AB, Chao J, Palmer LG. Tissue kallikrein activation of the epithelial Na channel. Am J Physiol Ren Physiol. 2012;303(4):F540–50.
El Moghrabi S, Houillier P, Picard N, Sohet F, Wootla B, Bloch-Faure M, et al. Tissue kallikrein permits early renal adaptation to potassium load. Proc Natl Acad Sci U S A. 2010;107(30):13526–31.
Svenningsen P, Bistrup C, Friis UG, Bertog M, Haerteis S, Krueger B, et al. Plasmin in nephrotic urine activates the epithelial sodium channel. J Am Soc Nephrol. 2009;20(2):299–310.
Zacchia M, Trepiccione F, Morelli F, Pani A, Capasso G. Nephrotic syndrome: new concepts in the pathophysiology of sodium retention. J Nephrol. 2008;21(6):836–42.
Zachar RM, Skjodt K, Marcussen N, Walter S, Toft A, Nielsen MR, et al. The Epithelial Sodium Channel gamma-Subunit Is Processed Proteolytically in Human Kidney. J Am Soc Nephrol 2014.
Rubera I, Loffing J, Palmer LG, Frindt G, Fowler-Jaeger N, Sauter D, et al. Collecting duct-specific gene inactivation of alphaENaC in the mouse kidney does not impair sodium and potassium balance. J Clin Invest. 2003;112(4):554–65.
Christensen BM, Perrier R, Wang Q, Zuber AM, Maillard M, Mordasini D, et al. Sodium and potassium balance depends on alphaENaC expression in connecting tubule. J Am Soc Nephrol. 2010;21(11):1942–51.
Frindt G, Palmer LG. Na channels in the rat connecting tubule. Am J Physiol Ren Physiol. 2004;286(4):F669–74.
Nesterov V, Dahlmann A, Krueger B, Bertog M, Loffing J, Korbmacher C. Aldosterone-dependent and -independent regulation of the epithelial sodium channel (ENaC) in mouse distal nephron. Am J Physiol Ren Physiol. 2012;303(9):F1289–99.
Loffing J, Pietri L, Aregger F, Bloch-Faure M, Ziegler U, Meneton P, et al. Differential subcellular localization of ENaC subunits in mouse kidney in response to high- and low-Na diets. Am J Physiol Ren Physiol. 2000;279(2):F252–8.
Campean V, Theilig F, Paliege A, Breyer M, Bachmann S. Key enzymes for renal prostaglandin synthesis: site-specific expression in rodent kidney (rat, mouse). Am J Physiol Ren Physiol. 2003;285(1):F19–32.
Fuson AL, Komlosi P, Unlap TM, Bell PD, Peti-Peterdi J. Immunolocalization of a microsomal prostaglandin E synthase in rabbit kidney. Am J Physiol Ren Physiol. 2003;285(3):F558–64.
Peti-Peterdi J, Komlosi P, Fuson AL, Guan Y, Schneider A, Qi Z, et al. Luminal NaCl delivery regulates facilitated renal Na + and water reabsorption. FASEB J 2007;21(13):3717-112(1):76-‐82.
Guan Y, Zhang Y, Breyer RM, Fowler B, Davis L, Hebert RL, et al. Prostaglandin E2 inhibits renal collecting duct Na + absorption by activating the EP1 receptor. J Clin Invest. 1998;102(1):194–201.
Hebert RL, Jacobson HR, Fredin D, Breyer MD. Evidence that separate PGE2 receptors modulate water and sodium transport in rabbit cortical collecting duct. Am J Physiol. 1993;265(5 Pt 2):F643–50.
Ando Y, Asano Y. Luminal prostaglandin E2 modulates sodium and water transport in rabbit cortical collecting ducts. Am J Physiol. 1995;268(6 Pt 2):F1093–101.
Sakairi Y, Jacobson HR, Noland TD, Breyer MD. Luminal prostaglandin E receptors regulate salt and water transport in rabbit cortical collecting duct. Am J Physiol. 1995;269(2 Pt 2):F257–65.
Jia Z, Zhang A, Zhang H, Dong Z, Yang T. Deletion of microsomal prostaglandin E synthase-1 increases sensitivity to salt loading and angiotensin II infusion. Circ Res. 2006;99(11):1243–51.
Ye W, Zhang H, Hillas E, Kohan DE, Miller RL, Nelson RD, et al. Expression and function of COX isoforms in renal medulla: evidence for regulation of salt sensitivity and blood pressure. Am J Physiol Ren Physiol. 2006;290(2):F542–9.
Chen J, Zhao M, He W, Milne GL, Howard JR, Morrow J, et al. Increased dietary NaCl induces renal medullary PGE2 production and natriuresis via the EP2 receptor. Am J Physiol Ren Physiol. 2008;295(3):F818–25.
Qi Z, Hao CM, Langenbach RI, Breyer RM, Redha R, Morrow JD, et al. Opposite effects of cyclooxygenase-1 and -2 activity on the pressor response to angiotensin II. J Clin Invest. 2002;110(1):61–9.
Kennedy CR, Zhang Y, Brandon S, Guan Y, Coffee K, Funk CD, et al. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat Med. 1999;5(2):217–20.
Lehrmann H, Thomas J, Kim SJ, Jacobi C, Leipziger J. Luminal P2Y2 receptor-mediated inhibition of Na + absorption in isolated perfused mouse CCD. J Am Soc Nephrol. 2002;13(1):10–8.
Pochynyuk O, Bugaj V, Rieg T, Insel PA, Mironova E, Vallon V, et al. Paracrine regulation of the epithelial Na + channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem. 2008;283(52):36599–607.
Kishore BK, Chou CL, Knepper MA. Extracellular nucleotide receptor inhibits AVP-stimulated water permeability in inner medullary collecting duct. Am J Physiol. 1995;269(6 Pt 2):F863–9.
Wildman SS, Boone M, Peppiatt-Wildman CM, Contreras-Sanz A, King BF, Shirley DG, et al. Nucleotides downregulate aquaporin 2 via activation of apical P2 receptors. J Am Soc Nephrol. 2009;20(7):1480–90.
Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, et al. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J. 2007;21(13):3717–26.
Terada Y, Knepper MA. Thiazide-sensitive NaCl absorption in rat cortical collecting duct. Am J Physiol. 1990;259(3 Pt 2):F519–28.
Leviel F, Hubner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, et al. The Na + -dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na + reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest. 2010;120(5):1627–35. This study demonstrates the presence of electroneutral, amiloride resistant, thiazide- sensitive transepithelial NaCl absorption in mouse CCDs.
Amasheh S, Milatz S, Krug SM, Bergs M, Amasheh M, Schulzke JD, et al. Na + absorption defends from paracellular back-leakage by claudin-8 upregulation. Biochem Biophys Res Commun. 2009;378(1):45–50.
Le Moellic C, Boulkroun S, Gonzalez-Nunez D, Dublineau I, Cluzeaud F, Fay M, et al. Aldosterone and tight junctions: modulation of claudin-4 phosphorylation in renal collecting duct cells. Am J Physiol Cell Physiol. 2005;289(6):C1513–21.
Sansom SC, O’Neil RG. Mineralocorticoid regulation of apical cell membrane Na + and K+ transport of the cortical collecting duct. Am J Physiol. 1985;248(6 Pt 2):F858–68.
Hou J, Renigunta A, Yang J, Waldegger S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc Natl Acad Sci U S A. 2010;107(42):18010–5.
Yamauchi K, Rai T, Kobayashi K, Sohara E, Suzuki T, Itoh T, et al. Disease-causing mutant WNK4 increases paracellular chloride permeability and phosphorylates claudins. Proc Natl Acad Sci U S A. 2004;101(13):4690–4.
Schambelan M, Sebastian A, Rector Jr FC. Mineralocorticoid-resistant renal hyperkalemia without salt wasting (type II pseudohypoaldosteronism): role of increased renal chloride reabsorption. Kidney Int. 1981;19(5):716–27.
Take C, Ikeda K, Kurasawa T, Kurokawa K. Increased chloride reabsorption as an inherited renal tubular defect in familial type II pseudohypoaldosteronism. N Engl J Med. 1991;324(7):472–6.
Kahle KT, Rinehart J, Ring A, Gimenez I, Gamba G, Hebert SC, et al. WNK protein kinases modulate cellular Cl- flux by altering the phosphorylation state of the Na-K-Cl and K-Cl cotransporters. Physiology (Bethesda). 2006;21:326–35.
Gong Y, Yu M, Yang J, Gonzales E, Perez R, Hou M, et al. The Cap1-claudin-4 regulatory pathway is important for renal chloride reabsorption and blood pressure regulation. Proc Natl Acad Sci U S A. 2014;111(36):E3766–74.
Wall SM, Hassell KA, Royaux IE, Green ED, Chang JY, Shipley GL, et al. Localization of pendrin in mouse kidney. Am J Physiol Ren Physiol. 2003;284(1):F229–41.
Kim YH, Kwon TH, Frische S, Kim J, Tisher CC, Madsen KM, et al. Immunocytochemical localization of pendrin in intercalated cell subtypes in rat and mouse kidney. Am J Physiol Ren Physiol. 2002;283(4):F744–54.
Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, et al. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci U S A. 2001;98(7):4221–6. This study identified the apical Cl−/HCO3− exchanger of β- intercalated cells as the pendrin gene product.
Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, et al. Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: role of pendrin in mineralocorticoid-induced hypertension. Hypertension. 2003;42(3):356–62. This study demonstrated that pendrin is critical in the pathogenesis of mineralocorticoid-induced hypertension.
Kim YH, Pech V, Spencer KB, Beierwaltes WH, Everett LA, Green ED, et al. Reduced ENaC protein abundance contributes to the lower blood pressure observed in pendrin-null mice. Am J Physiol Ren Physiol. 2007;293(4):F1314–24.
Wall SM, Kim YH, Stanley L, Glapion DM, Everett LA, Green ED, et al. NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: role in Cl- conservation. Hypertension. 2004;44(6):982–7.
Jacques T, Picard N, Miller RL, Riemondy KA, Houillier P, Sohet F, et al. Overexpression of pendrin in intercalated cells produces chloride-sensitive hypertension. J Am Soc Nephrol. 2013;24(7):1104–13. This study showed that pendrin overexpression in ICs results in increased sodium chloride absorption in the CD and salt-sensitive elevation of blood pressure. The pressor effect of high salt intake was strictly chloride-dependent, and occurred despite appropriate downregulation of the sodium transporters in the ASDN.
Gueutin V, Vallet M, Jayat M, Peti-Peterdi J, Corniere N, Leviel F, et al. Renal beta-intercalated cells maintain body fluid and electrolyte balance. J Clin Invest. 2013;123(10):4219–31. This study showed that when the B1-subunit of the H+-ATPase is deleted in mouse, a syndrome of mild distal renal tubular acidosis (RTA) develops which, like the human syndrome, is associated with renal loss of NaCl, K+ and water causing hypovolemia, hypokalemia and polyuria. Sodium wasting was due to a reduction of sodium absorption by the principal cells adjacent to the affected intercalated cells, in addition to the reduction of sodium chloride absorption by the β-intercalated cell itself. The authors discovered that the genetic or pharmacological inactivation of the proton pump caused ATP secretion by β-intercalated cells, which in turn triggers PGE2 release by acting on luminal calcium-coupled purinergic receptors, resulting in inhibition of ENaC in the neighboring PCs.
Chambrey R, Kurth I, Peti-Peterdi J, Houillier P, Purkerson JM, Leviel F, et al. Renal intercalated cells are rather energized by a proton than a sodium pump. Proc Natl Acad Sci U S A. 2013;110(19):7928–33. This study shows that the H+‐ ATPase in β-intercalated cells is the major driver of secondary active transport, rather than the Na+,K+ ATPase. This unique feature is the cornerstone for NaCl transport in these cells.
Nissant A, Paulais M, Lachheb S, Lourdel S, Teulon J. Similar chloride channels in the connecting tubule and cortical collecting duct of the mouse kidney. Am J Physiol Ren Physiol. 2006;290(6):F1421–9.
Schultheis PJ, Lorenz JN, Meneton P, Nieman ML, Riddle TM, Flagella M, et al. Phenotype resembling Gitelman’s syndrome in mice lacking the apical Na + −Cl-cotransporter of the distal convoluted tubule. J Biol Chem. 1998;273(44):29150–5.
Loffing J, Vallon V, Loffing-Cueni D, Aregger F, Richter K, Pietri L, et al. Altered renal distal tubule structure and renal Na(+) and Ca(2+) handling in a mouse model for Gitelman’s syndrome. J Am Soc Nephrol. 2004;15(9):2276–88.
Nijenhuis T, Hoenderop JG, Loffing J, van der Kemp AW, van Os CH, Bindels RJ. Thiazide-induced hypocalciuria is accompanied by a decreased expression of Ca2+ transport proteins in kidney. Kidney Int. 2003;64(2):555–64.
Vallet M, Picard N, Loffing-Cueni D, Fysekidis M, Bloch-Faure M, Deschenes G, et al. Pendrin regulation in mouse kidney primarily is chloride-dependent. J Am Soc Nephrol. 2006;17(8):2153–63.
Soleimani M, Barone S, Xu J, Shull GE, Siddiqui F, Zahedi K, et al. Double knockout of pendrin and Na-Cl cotransporter (NCC) causes severe salt wasting, volume depletion, and renal failure. Proc Natl Acad Sci U S A. 2012;109(33):13368–73.
Morris RG, Hoorn EJ, Knepper MA. Hypokalemia in a mouse model of Gitelman’s syndrome. Am J Physiol Ren Physiol. 2006;290(6):F1416–20.
Hadchouel J, Soukaseum C, Busst C, Zhou XO, Baudrie V, Zurrer T, et al. Decreased ENaC expression compensates the increased NCC activity following inactivation of the kidney-specific isoform of WNK1 and prevents hypertension. Proc Natl Acad Sci U S A. 2010;107(42):18109–14.
Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, et al. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet. 2006;38(10):1124–32.
Castaneda-Bueno M, Cervantes-Perez LG, Vazquez N, Uribe N, Kantesaria S, Morla L, et al. Activation of the renal Na+:Cl- cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci U S A. 2012;109(20):7929–34.
Ohno M, Uchida K, Ohashi T, Nitta K, Ohta A, Chiga M, et al. Immunolocalization of WNK4 in mouse kidney. Histochem Cell Biol. 2011;136(1):25–35.
Shibata S, Rinehart J, Zhang J, Moeckel G, Castaneda-Bueno M, Stiegler AL, et al. Mineralocorticoid receptor phosphorylation regulates ligand binding and renal response to volume depletion and hyperkalemia. Cell Metab. 2013;18(5):660–71.
Pech V, Pham TD, Hong S, Weinstein AM, Spencer KB, Duke BJ, et al. Pendrin modulates ENaC function by changing luminal HCO3. J Am Soc Nephrol. 2010;21(11):1928–41.
Pech V, Zheng W, Pham TD, Verlander JW, Wall SM. Angiotensin II activates H + −ATPase in type A intercalated cells. J Am Soc Nephrol. 2008;19(1):84–91.
Wagner CA, Mohebbi N, Uhlig U, Giebisch GH, Breton S, Brown D, et al. Angiotensin II stimulates H(+)-ATPase activity in intercalated cells from isolated mouse connecting tubules and cortical collecting ducts. Cell Physiol Biochem. 2011;28(3):513–20.
Pech V, Kim YH, Weinstein AM, Everett LA, Pham TD, Wall SM. Angiotensin II increases chloride absorption in the cortical collecting duct in mice through a pendrin-dependent mechanism. Am J Physiol Ren Physiol. 2007;292(3):F914–F9200000.
Deetjen P, Thomas J, Lehrmann H, Kim SJ, Leipziger J. The luminal P2Y receptor in the isolated perfused mouse cortical collecting duct. J Am Soc Nephrol. 2000;11(10):1798–806.
Sipos A, Vargas SL, Toma I, Hanner F, Willecke K, Peti-Peterdi J. Connexin 30 deficiency impairs renal tubular ATP release and pressure natriuresis. J Am Soc Nephrol. 2009;20(8):1724–32.
Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev. 2009;89(1):193–277.
McCulloch F, Chambrey R, Eladari D, Peti-Peterdi J. Localization of connexin 30 in the luminal membrane of cells in the distal nephron. Am J Physiol Ren Physiol. 2005;289(6):F1304–12.
Hanner F, Lam L, Nguyen MT, Yu A, Peti-Peterdi J. Intrarenal localization of the plasma membrane ATP channel pannexin1. Am J Physiol Ren Physiol. 2012;303(10):F1454–9.
Mironova E, Peti-Peterdi J, Bugaj V, Stockand JD. Diminished paracrine regulation of the epithelial Na + channel by purinergic signaling in mice lacking connexin 30. J Biol Chem. 2011;286(2):1054–60.
Welch BD, Carlson NG, Shi H, Myatt L, Kishore BK. P2Y2 receptor stimulated release of prostaglandin E2 by rat inner medullary collecting duct preparations. Am J Physiol Ren Physiol. 2003;285(4):F711–21.
Murakami M, Nakashima K, Kamei D, Masuda S, Ishikawa Y, Ishii T, et al. Cellular prostaglandin E2 production by membrane-bound prostaglandin E synthase-2 via both cyclooxygenases-1 and -2. J Biol Chem. 2003;278(39):37937–47.
Yang G, Chen L, Zhang Y, Zhang X, Wu J, Li S, et al. Expression of mouse membrane-associated prostaglandin E2 synthase-2 (mPGES-2) along the urogenital tract. Biochim Biophys Acta. 2006;1761(12):1459–68.
Liu Y, Flores D, Carrisoza-Gaytan R, Rohatgi R. Biomechanical regulation of cyclooxygenase-2 in the renal collecting duct. Am J Physiol Ren Physiol. 2014;306(2):F214–23.
Flores D, Liu Y, Liu W, Satlin LM, Rohatgi R. Flow-induced prostaglandin E2 release regulates Na and K transport in the collecting duct. Am J Physiol Ren Physiol. 2012;303(5):F632–8.
Yang T, Schnermann JB, Briggs JP. Regulation of cyclooxygenase-2 expression in renal medulla by tonicity in vivo and in vitro. Am J Physiol. 1999;277(1 Pt 2):F1–9.
Acknowledgments
The authors thank Juliette Hadchouel and Dominique Eladari for critical reading of the manuscript. Thanks to all members of the laboratory for contributing to the research projects. RC is funded by the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Fondation pour la Recherche Médicale, the Société de Néphrologie, and l’Agence Nationale de la Recherche Projet ANR-12-BSV1-0017. FT is funded by ERA-EDTA Long Term Fellowship–LTF 141-2013.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Régine Chambrey and Francesco Trepiccione declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Hypertension and the Kidney
Rights and permissions
About this article
Cite this article
Chambrey, R., Trepiccione, F. Relative Roles of Principal and Intercalated Cells in the Regulation of Sodium Balance and Blood Pressure. Curr Hypertens Rep 17, 27 (2015). https://doi.org/10.1007/s11906-015-0538-0
Published:
DOI: https://doi.org/10.1007/s11906-015-0538-0