Abstract
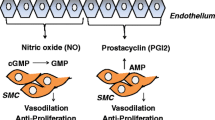
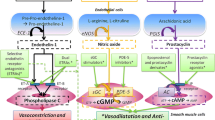
Pulmonary arterial hypertension (PAH) is a rare, progressively worsening disease characterized by dysfunction among endothelial and smooth muscle cells within the pulmonary vasculature with a resultant increase in pulmonary vascular resistance, right ventricular maladaptation and failure, and ultimately early death. The three major therapeutic classes of medications available to treat PAH act as either prostacyclin analogs or endothelin receptor antagonists (ERAs) or by increasing local nitric oxide (NO) levels by means of phosphodiesterase type 5 inhibitors. Several recent trials have investigated the use of oral prostanoid therapy, next-generation ERAs, and soluble guanylate cyclase stimulators (to increase NO levels) as well as novel formulations of pre-existing therapies. The goal of this manuscript is to briefly review established therapies and then discuss recent developments and practical considerations in each of the major drug classes.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–65.
Waxman AB, Zamanian RT. Pulmonary arterial hypertension: new insights into the optimal role of current and emerging prostacyclin therapies. Am. J. Cardiol. 2013;111:1A–16A; quiz 17A–19A.
Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334:296–301.
Sitbon O, Humbert M, Nunes H, Parent F, Garcia G, Hervé P, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40:780–8.
McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension the impact of epoprostenol therapy. Circulation. 2002;106:1477–82.
Agarwal R, Gomberg-Maitland M. Current therapeutics and practical management strategies for pulmonary arterial hypertension. Am Heart J. 2011;162:201–13.
Chin KM, Badesch DB, Robbins IM, Tapson VF, Palevsky HI, Kim NH, et al. Two formulations of epoprostenol sodium in the treatment of pulmonary arterial hypertension: EPITOME-1 (epoprostenol for injection in pulmonary arterial hypertension), a phase IV, open-label, randomized study. Am Heart J. 2014;167:218–225.e1.
Sitbon O, Delcroix M, Bergot E, Boonstra AB, Granton J, Langleben D, et al. EPITOME-2: an open-label study assessing the transition to a new formulation of intravenous epoprostenol in patients with pulmonary arterial hypertension. Am Heart J. 2014;167:210–7.
Tamura Y, Ono T, Fukuda K, Satoh T, Sasayama S. Evaluation of a new formulation of epoprostenol sodium in Japanese patients with pulmonary arterial hypertension (EPITOME4). Adv Ther. 2013;30:459–71. The Epitome-1, 2, and 4 trials demonstrate that Veletri is similar in efficacy and safety when compared to Flolan and has higher treatment satisfaction. These studies may motivate providers to transition patients from Flolan, one of the most established treatments in PAH, to Veletri.
Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Bourge RC, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165:800–4.
Barst RJ, Galie N, Naeije R, Simonneau G, Jeffs R, Arneson C, et al. Long-term outcome in pulmonary arterial hypertension patients treated with subcutaneous treprostinil. Eur Respir J. 2006;28:1195–203.
Minai OA, Parambil J, Dweik RA, Davila GH, Peterson L, Rollins KD, et al. Impact of switching from epoprostenol to IV treprostinil on treatment satisfaction and quality of life in patients with pulmonary hypertension. Respir Med. 2013;107:458–65.
Gomberg-Maitland M, Tapson VF, Benza RL, McLaughlin VV, Krichman A, Widlitz AC, et al. Transition from intravenous epoprostenol to intravenous treprostinil in pulmonary hypertension. Am J Respir Crit Care Med. 2005;172:1586–9.
Sitbon O, Manes A, Jais X, Pallazini M, Humbert M, Presotto L, et al. Rapid switch from intravenous epoprostenol to intravenous treprostinil in patients with pulmonary arterial hypertension. J Cardiovasc Pharmacol. 2007;49:1–5.
Tapson VF, Gomberg-Maitland M, McLaughlin VV, Benza RL, Widlitz AC, Krichman A, et al. Safety and efficacy of IV treprostinil for pulmonary arterial hypertension: a prospective, multicenter, open-label, 12-week trial. Chest J. 2006;129:683–8.
Hiremath J, Thanikachalam S, Parikh K, Shanmugasundaram S, Bangera S, Shapiro L, et al. Exercise improvement and plasma biomarker changes with intravenous treprostinil therapy for pulmonary arterial hypertension: a placebo-controlled trial. J Heart Lung Transplant. 2010;29:137–49.
McLaughlin VV, Benza RL, Rubin LJ, Channick RN, Voswinckel R, Tapson VF, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol. 2010;55:1915–22.
Olschewski H, Simonneau G, Galiè N, Higenbottam T, Naeije R, Rubin LJ, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347:322–9.
Opitz CF, Wensel R, Winkler J, Halank M, Bruch L, Kleber F-X, et al. Clinical efficacy and survival with first-line inhaled iloprost therapy in patients with idiopathic pulmonary arterial hypertension. Eur Heart J. 2005;26:1895–902.
Wu Y, O’Callaghan DS, Humbert M. An update on medical therapy for pulmonary arterial hypertension. Curr Hypertens Rep. 2013;15:614–22.
Galiè N, Humbert M, Vachiéry J-L, Vizza CD, Kneussl M, Manes A, et al. Effects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol. 2002;39:1496–502.
Barst RJ, McGoon M, McLaughlin V, Tapson V, Rich S, Rubin L, et al. Beraprost therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2003;41:2119–25.
Jing Z-C, Parikh K, Pulido T, Jerjes-Sanchez C, White RJ, Allen R, et al. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation. 2013;127:624–33. This trial demonstrated a modest but significant improvement in 6MWD, its primary endpoint. Additional trials are currently underway to assess the efficacy of oral treprostinil when used in combination with other agents.
Tapson VF, Torres F, Kermeen F, Keogh AM, Allen RP, Frantz RP, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trial. Chest. 2012;142:1383–90.
Tapson VF, Jing Z-C, Xu K-F, Pan L, Feldman J, Kiely DG, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trial. Chest. 2013;144:952–8.
Simonneau G, Torbicki A, Hoeper MM, Delcroix M, Karlócai K, Galiè N, et al. Selexipag: an oral, selective prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension. Eur Respir J. 2012;40:874–80. This small, phase II trial demonstrated the efficacy and safety of oral selexipag and motivated the larger phase III GRIPHON trial that has recently been completed and still awaits publication. Preliminary results show that the primary endpoint was met, which may lead to an FDA approval and potentially a new popular therapeutic option.
Selexipag meets primary endpoint in pivotal phase III GRIPHON outcome study in patients with pulmonary arterial hypertension <ATLN.VX>. Reuters [Internet]. 2014 Jun 15 [cited 2014 Jul 10]; Available from: http://www.reuters.com/article/2014/06/15/idUSnHUGn8QMsa+1cc+ONE20140615.
Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–5.
Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–9.
Rubens C, Ewert R, Halank M, Wensel R, Orzechowski H-D, Schultheiss H-P, et al. Big endothelin-1 and endothelin-1 plasma levels are correlated with the severity of primary pulmonary hypertension*. Chest J. 2001;120:1562–9.
Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358:1119–23.
Galiè N, Rubin L, Hoeper M, Jansa P, Al-Hiti H, Meyer G, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet. 2008;371:2093–100.
McLaughlin VV, Sitbon O, Badesch DB, Barst RJ, Black C, Galiè N, et al. Survival with first-line bosentan in patients with primary pulmonary hypertension. Eur Respir J. 2005;25:244–9.
Humbert M, Segal ES, Kiely DG, Carlsen J, Schwierin B, Hoeper MM. Results of European post-marketing surveillance of bosentan in pulmonary hypertension. Eur Respir J. 2007;30:338–44.
Commissioner O of the Safety Information—Tracleer (bosentan) tablets [Internet]. [cited 2014 Jul 16]. Available from: http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm327930.htm.
Ben-Yehuda O, Pizzuti D, Brown A, Littman M, Gillies H, Henig N, et al. Long-term hepatic safety of ambrisentan in patients with pulmonary arterial hypertension. J Am Coll Cardiol. 2012;60:80–1.
Galiè N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, et al. Ambrisentan for the treatment of pulmonary arterial hypertension results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117:3010–9.
Pulido T, Adzerikho I, Channick RN, Delcroix M, Galiè N, Ghofrani H-A, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369:809–18. A landmark trial that used morbidity and mortality as a composite primary endpoint. The results led to the approval of macitentan.
Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–57.
Rubin LJ, Badesch DB, Fleming TR, Galiè N, Simonneau G, Ghofrani HA, et al. Long-term treatment with sildenafil citrate in pulmonary arterial hypertension: the super-2 study. Chest J. 2011;140:1274–83.
Galiè N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–903.
Lang M, Kojonazarov B, Tian X, Kalymbetov A, Weissmann N, Grimminger F, et al. The soluble guanylate cyclase stimulator riociguat ameliorates pulmonary hypertension induced by hypoxia and SU5416 in rats. PLoS One. 2012;7:e43433.
Ghofrani H-A, Galiè N, Grimminger F, Grünig E, Humbert M, Jing Z-C, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369:330–40.
Ghofrani H-A, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369:319–29. The above two trials by Ghofrani et al. demonstrated the efficacy of Riociguat, a therapy in a novel class, in both PAH and CTEPH. It is the only therapy approved by the FDA for the treatment of inoperable CTEPH.
Jing Z-C, Yu Z-X, Shen J-Y, Wu B-X, Xu K-F, Zhu X-Y, et al. Vardenafil in pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med. 2011;183:1723–9. This small study demonstrated the efficacy of Vardenafil. Additional studies are needed to demonstrate if Vardenafil is more efficacious than existing PDE-5 inhibitors.
Fan Y-F, Zhang R, Jiang X, Wen L, Wu D-C, Liu D, et al. The phosphodiesterase-5 inhibitor vardenafil reduces oxidative stress while reversing pulmonary arterial hypertension. Cardiovasc Res. 2013;99:395–403.
Nazzareno Galie, Dieter Neuser, Katharina Muller, Andrea-Viviana Scalise, Ekkehard Grunig. A placebo-controlled, double-blind phase ii interaction study to evaluate blood pressure following addition of riociguat to patients with symptomatic pulmonary arterial hypertension (pah) receiving sildenafil (PATENT PLUS). B98 Clin. TRIALS Pulm. Hypertens. [Internet]. American Thoracic Society; 2013 [cited 2014 Jul 22]. p. A3530–A3530. Available from: http://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2013.187.1_MeetingAbstracts.A3530.
Taichman DB, Ornelas J, Chung L, Klinger J, Lewis S, Mandel J, et al. Pharmacological therapy for pulmonary arterial hypertension in adults: CHEST guideline. CHEST J. [Internet]. 2014 [cited 2014 Jul 25]; Available from: doi:10.1378/chest.14-0793.
Galié N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, et al. Updated treatment algorithm of pulmonary arterial hypertension. J. Am. Coll. Cardiol. [Internet]. 2013 [cited 2014 Jul 25];62. Available from: doi:10.1016/j.jacc.2013.10.031.
Galié N, Manes A. New treatment strategies for pulmonary arterial hypertension hopes or hypes? J Am Coll Cardiol. 2013;62:1101–2.
Compliance with Ethics Guidelines
Conflict of Interest
Aaron M. Wolfson and Nathaniel Steiger declare no conflict of interest. Mardi Gomberg-Maitland has received funding for clinical trials from Actelion, Gilead, Medtronic, Novartis, Lung Biotechnology, Reata, and Ventripoint; she has served as a consultant for Actelion, Bayer, Gilead, Medtronic, Merck, Bellerophon, and United Therapeutics; she has received honoraria for CME from Medscape and ABComm.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Pulmonary Hypertension
Rights and permissions
About this article
Cite this article
Wolfson, A.M., Steiger, N. & Gomberg-Maitland, M. New Pharmacotherapies for Pulmonary Hypertension: Where Do They Fit in?. Curr Hypertens Rep 16, 496 (2014). https://doi.org/10.1007/s11906-014-0496-y
Published:
DOI: https://doi.org/10.1007/s11906-014-0496-y