Abstract
Purpose of Review
Telomere biology disorders (TBDs) are germline-inherited conditions characterized by reduction in telomerase function, accelerated shortening of telomeres, predisposition to organ-failure syndromes, and increased risk of neoplasms, especially myeloid malignancies. In normal cells, critically short telomeres trigger apoptosis and/or cellular senescence. However, the evolutionary mechanism by which TBD-related telomerase-deficient cells can overcome this fitness constraint remains elusive.
Recent Findings
Preliminary data suggests the existence of adaptive somatic mosaic states characterized by variants in TBD-related genes and maladaptive somatic mosaic states that attempt to overcome hematopoietic fitness constraints by alternative methods leading to clonal hematopoiesis.
Summary
TBDs are both rare and highly heterogeneous in presentation, and the association of TBD with malignant transformation is unclear. Understanding the clonal complexity and mechanisms behind TBD-associated molecular signatures that lead to somatic adaptation in the setting of defective hematopoiesis will help inform therapy and treatment for this set of diseases.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
McNally EJ, Luncsford PJ, Armanios M. Long telomeres and cancer risk: the price of cellular immortality. J Clin Invest. 2019;129(9):3474–81.
Blackburn EH, Gall JG. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol. 1978;120(1):33–53.
Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in tetrahymena extracts. Cell. 1985;43(2, Part 1):405–13.
Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12(10):1133–8.
O’Sullivan RJ, Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol. 2010;11(3):171–81.
Harley CB. Telomere loss: mitotic clock or genetic time bomb? Mutat Res/DNAging. 1991;256(2):271–82.
Greider CW. Telomerase is processive. Mol Cell Biol. 1991;11(9):4572–80.
Cesare AJ, Reddel RR. Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet. 2010;11(5):319–30.
Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25(3):585–621.
van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509(7501):439–46.
Blackburn EH, Epel ES, Lin J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350(6265):1193–8.
Dunham MA, Neumann AA, Fasching CL, Reddel RR. Telomere maintenance by recombination in human cells. Nat Genet. 2000;26(4):447–50.
Gravekamp C, Chandra D. Aging and cancer vaccines. Crit Rev Oncog. 2013;18(6):585–95.
Vulliamy TJ, Knight SW, Mason PJ, Dokal I. Very short telomeres in the peripheral blood of patients with X-linked and autosomal dyskeratosis congenita. Blood Cells Mol Dis. 2001;27(2):353–7.
Bertuch AA. The molecular genetics of the telomere biology disorders. RNA Biol. 2016;13(8):696–706.
Savage SA, Bertuch AA. The genetics and clinical manifestations of telomere biology disorders. Genet Med. 2010;12(12):753–64.
Shay JW, Wright WE. Telomeres and telomerase: three decades of progress. Nat Rev Genet. 2019;20(5):299–309.
Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356(13):1317–26.
Feurstein S, Adegunsoye A, Mojsilovic D, Vij R, West DePersia AH, Rajagopal PS, et al. Telomere biology disorder prevalence and phenotypes in adults with familial hematologic and/or pulmonary presentations. Blood Adv. 2020;4(19):4873–86.
Mangaonkar AA, Patnaik MM. Short telomere syndromes in clinical practice: bridging bench and bedside. Mayo Clin Proc. 2018;93(7):904–16.
Alter BP, Rosenberg PS, Giri N, Baerlocher GM, Lansdorp PM, Savage SA. Telomere length is associated with disease severity and declines with age in dyskeratosis congenita. Haematologica. 2012;97(3):353–9.
Blanche PA, Neelam G, Sharon AS, Philip SR. Cancer in the National Cancer Institute inherited bone marrow failure syndrome cohort after fifteen years of follow-up. Haematologica. 2018;103(1):30–9.
Schratz KE, Haley L, Danoff SK, Blackford AL, DeZern AE, Gocke CD, et al. Cancer spectrum and outcomes in the Mendelian short telomere syndromes. Blood. 2020;135(22):1946–56. Description of the clinical outcomes of the TBD cohort assembled at Johns Hopkins. First report indicating that TBD patients present with increased CH and a different mutational signature compared to ARCH.
Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in the National Cancer Institute inherited bone marrow failure syndrome cohort after fifteen years of follow-up. Haematologica. 2018;103(1):30–9. Description of the clinical outcomes of the TBD cohort assembled by the NIH describing higher risk to develop MN and specific characteristics of these patients.
Schratz KE, Armanios M. Cancer and myeloid clonal evolution in the short telomere syndromes. Curr Opin Genet Dev. 2020;60:112–8.
Jaiswal S, Ebert BL. Clonal hematopoiesis in human aging and disease. Science. 2019;366(6465):eaan4673.
Fabre MA, de Almeida JG, Fiorillo E, Mitchell E, Damaskou A, Rak J, et al. The longitudinal dynamics and natural history of clonal haematopoiesis. Nature. 2022;606(7913):335–42.
Mitchell E, Spencer Chapman M, Williams N, Dawson KJ, Mende N, Calderbank EF, et al. Clonal dynamics of haematopoiesis across the human lifespan. Nature. 2022;606(7913):343–50.
Kusne Y, Xie Z, Patnaik MM. Clonal hematopoiesis: molecular and clinical implications. Leuk Res. 2022;113:106787.
Gregory JJ Jr, Wagner JE, Verlander PC, Levran O, Batish SD, Eide CR, et al. Somatic mosaicism in Fanconi anemia: evidence of genotypic reversion in lymphohematopoietic stem cells. Proc Natl Acad Sci USA. 2001;98(5):2532–7.
Myers KC, Furutani E, Weller E, Siegele B, Galvin A, Arsenault V, et al. Clinical features and outcomes of patients with Shwachman-Diamond syndrome and myelodysplastic syndrome or acute myeloid leukaemia: a multicentre, retrospective, cohort study. Lancet Haematol. 2020;7(3):e238–46.
Gutierrez-Rodrigues F, Groarke EM, Clé DV, Patel BA, Donaires FS, Spitofsky N, et al. Clonal hematopoiesis in telomere biology disorders associates with the underlying germline defect and somatic mutations in POT1, PPM1D, and TERT promoter. Blood. 2021;138(Supplement 1):1111-. Description in more detail of the somatic mutational landscape observed in the larger number of TBD patients to date. Possible association between specific somatic events and germline mutations.
Ferrer A, Mangaonkar AA, Patnaik MM. Clonal hematopoiesis and myeloid neoplasms in the context of telomere biology disorders. Curr Hematol Malig Rep. 2022;17(3):61–8.
Pritzl SL, Gurney M, Badar T, Ferrer A, Lasho T, Finke C, et al. Clinical and molecular spectrum and prognostic outcomes of U2AF1 mutant clonal hematopoiesis- a prospective mayo clinic cohort study. Leuk Res. 2023;125:107007.
Schratz KE, Gaysinskaya V, Cosner ZL, DeBoy EA, Xiang Z, Kasch-Semenza L, et al. Somatic reversion impacts myelodysplastic syndromes and acute myeloid leukemia evolution in the short telomere disorders. J Clin Invest. 2021;131(18). Study of genetic rescue in the development of MDS/AML in TBD patients.
Maryoung L, Yue Y, Young A, Newton CA, Barba C, van Oers NS, et al. Somatic mutations in telomerase promoter counterbalance germline loss-of-function mutations. J Clin Invest. 2017;127(3):982–6.
Revy P, Kannengiesser C, Bertuch AA. Genetics of human telomere biology disorders. Nat Rev Genet. 2023;24(2):86–108.
Vaziri H, Dragowska W, Allsopp RC, Thomas TE, Harley CB, Lansdorp PM. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc Natl Acad Sci USA. 1994;91(21):9857–60.
Armanios M, Alder JK, Parry EM, Karim B, Strong MA, Greider CW. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am J Human Genet. 2009;85(6):823–32.
d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426(6963):194–8.
Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol. 2003;13(17):1549–56.
Jacobs JJ, de Lange T. Significant role for p16INK4a in p53-independent telomere-directed senescence. Curr Biol. 2004;14(24):2302–8.
Sfeir A, de Lange T. Removal of shelterin reveals the telomere end-protection problem. Science. 2012;336(6081):593–7.
Lazzerini-Denchi E, Sfeir A. Stop pulling my strings — what telomeres taught us about the DNA damage response. Nat Rev Mol Cell Biol. 2016;17(6):364–78.
Kiss T, Fayet-Lebaron E, Jády BE. Box H/ACA small ribonucleoproteins. Mol Cell. 2010;37(5):597–606.
Darzacq X, Kittur N, Roy S, Shav-Tal Y, Singer RH, Meier UT. Stepwise RNP assembly at the site of H/ACA RNA transcription in human cells. J Cell Biol. 2006;173(2):207–18.
Schmidt JC, Cech TR. Human telomerase: biogenesis, trafficking, recruitment, and activation. Genes Dev. 2015;29(11):1095–105.
Roake CM, Artandi SE. Regulation of human telomerase in homeostasis and disease. Nat Rev Mol Cell Biol. 2020;21(7):384–97.
Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279(5349):349–52.
Chiba K, Lorbeer FK, Shain AH, McSwiggen DT, Schruf E, Oh A, et al. Mutations in the promoter of the telomerase gene TERT contribute to tumorigenesis by a two-step mechanism. Science. 2017;357(6358):1416–20.
Gutierrez-Rodrigues F, Donaires FS, Pinto A, Vicente A, Dillon LW, Clé DV, et al. Pathogenic TERT promoter variants in telomere diseases. Genet Med. 2019;21(7):1594–602.
Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci. 2007;104(18):7552–7.
Du H-Y, Pumbo E, Manley P, Field JJ, Bayliss SJ, Wilson DB, et al. Complex inheritance pattern of dyskeratosis congenita in two families with 2 different mutations in the telomerase reverse transcriptase gene. Blood. 2008;111(3):1128–30.
Aspesi A, Vallero S, Rocci A, Pavesi E, Lanciotti M, Ramenghi U, et al. Compound heterozygosity for two new TERT mutations in a patient with aplastic anemia. Pediatr Blood Cancer. 2010;55(3):550–3.
Gramatges MM, Qi X, Sasa GS, Chen JJL, Bertuch AA. A homozygous telomerase T-motif variant resulting in markedly reduced repeat addition processivity in siblings with Hoyeraal Hreidarsson syndrome. Blood. 2013;121(18):3586–93.
Niaz A, Truong J, Manoleras A, Fox LC, Blombery P, Vasireddy RS, et al. Functional interaction between compound heterozygous TERT mutations causes severe telomere biology disorder. Blood Adv. 2022;6(12):3779–91.
Çepni E, Satkın NB, Moheb LA, Rocha ME, Kayserili H. Biallelic TERT variant leads to Hoyeraal-Hreidarsson syndrome with additional dyskeratosis congenita findings. Am J Med Genet A. 2022;188(4):1226–32.
Stockklausner C, Raffel S, Klermund J, Bandapalli OR, Beier F, Brümmendorf TH, et al. A novel autosomal recessive TERT T1129P mutation in a dyskeratosis congenita family leads to cellular senescence and loss of CD34+ hematopoietic stem cells not reversible by mTOR-inhibition. Aging (Albany NY). 2015;7(11):911.
Marrone A, Walne A, Tamary H, Masunari Y, Kirwan M, Beswick R, et al. Telomerase reverse-transcriptase homozygous mutations in autosomal recessive dyskeratosis congenita and Hoyeraal-Hreidarsson syndrome. Blood. 2007;110(13):4198–205.
Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason PJ, et al. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413(6854):432–5.
Nguyen THD, Tam J, Wu RA, Greber BJ, Toso D, Nogales E, et al. Cryo-EM structure of substrate-bound human telomerase holoenzyme. Nature. 2018;557(7704):190–5.
Heiss NS, Knight SW, Vulliamy TJ, Klauck SM, Wiemann S, Mason PJ, et al. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998;19(1):32–8.
Knight SW, Heiss NS, Vulliamy TJ, Greschner S, Stavrides G, Pai GS, et al. X-linked dyskeratosis congenita is predominantly caused by missense mutations in the DKC1 gene. Am J Human Genet. 1999;65(1):50–8.
Walne AJ, Vulliamy T, Marrone A, Beswick R, Kirwan M, Masunari Y, et al. Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations in the telomerase-associated protein NOP10. Hum Mol Genet. 2007;16(13):1619–29.
Vulliamy T, Beswick R, Kirwan M, Marrone A, Digweed M, Walne A, et al. Mutations in the telomerase component NHP2 cause the premature ageing syndrome dyskeratosis congenita. Proc Natl Acad Sci. 2008;105(23):8073–8.
Benyelles M, O’Donohue M-F, Kermasson L, Lainey E, Borie R, Lagresle-Peyrou C, et al. NHP2 deficiency impairs rRNA biogenesis and causes pulmonary fibrosis and Høyeraal-Hreidarsson syndrome. Hum Mol Genet. 2020;29(6):907–22.
Zhong F, Savage SA, Shkreli M, Giri N, Jessop L, Myers T, et al. Disruption of telomerase trafficking by TCAB1 mutation causes dyskeratosis congenita. Genes Dev. 2011;25(1):11–6.
Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–34.
Morrison SJ, Prowse KR, Ho P, Weissman IL. Telomerase activity in hematopoietic cells is associated with self-renewal potential. Immunity. 1996;5(3):207–16.
Chiu C-P, Dragowska W, Kim NW, Vaziri H, Yui J, Thomas TE, et al. Differential expression of telomerase activity in hematopoietic progenitors from adult human bone marrow. Stem Cells. 1996;14(2):239–48.
Harel I, Benayoun BA, Machado B, Singh PP, Hu CK, Pech MF, et al. A platform for rapid exploration of aging and diseases in a naturally short-lived vertebrate. Cell. 2015;160(5):1013–26.
Meier B, Clejan I, Liu Y, Lowden M, Gartner A, Hodgkin J, et al. trt-1 is the Caenorhabditis elegans catalytic subunit of telomerase. PLoS Genet. 2006;2(2): e18.
Blasco MA, Lee H-W, Hande MP, Samper E, Lansdorp PM, DePinho RA, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91(1):25–34.
Lee HW, Blasco MA, Gottlieb GJ, Horner Ii JW, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392(6676):569–74.
Rudolph KL, Chang S, Lee H-W, Blasco M, Gottlieb GJ, Greider C, et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96(5):701–12.
Herrera E, Samper E, Martín-Caballero J, Flores JM, Lee HW, Blasco MA. Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. EMBO J. 1999;18(11):2950–60.
Niewisch MR, Giri N, McReynolds LJ, Alsaggaf R, Bhala S, Alter BP, et al. Disease progression and clinical outcomes in telomere biology disorders. Blood. 2022;139(12):1807–19.
Alder JK, Armanios M. Telomere-mediated lung disease. Physiol Rev. 2022;102(4):1703–20.
Walne AJ, Dokal I. Advances in the understanding of dyskeratosis congenita. Br J Haematol. 2009;145(2):164–72.
Armanios M. The role of telomeres in human disease. Annu Rev Genomics Hum Genet. 2022;23:363–81.
Rossiello F, Jurk D, Passos JF, di d’Adda Fagagna F. Telomere dysfunction in ageing and age-related diseases. Nat Cell Biol. 2022;24(2):135–47.
Knight S, Vulliamy T, Copplestone A, Gluckman E, Mason P, Dokal I. Dyskeratosis Congenita (DC) Registry: identification of new features of DC. Br J Haematol. 1998;103(4):990–6.
Alder JK, Cogan JD, Brown AF, Anderson CJ, Lawson WE, Lansdorp PM, et al. Ancestral mutation in telomerase causes defects in repeat addition processivity and manifests as familial pulmonary fibrosis. PLoS Genet. 2011;7(3):e1001352.
Armanios M, Chen JL, Chang YP, Brodsky RA, Hawkins A, Griffin CA, et al. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc Natl Acad Sci USA. 2005;102(44):15960–4.
Parry EM, Alder JK, Qi X, Chen JJ, Armanios M. Syndrome complex of bone marrow failure and pulmonary fibrosis predicts germline defects in telomerase. Blood. 2011;117(21):5607–11.
Takai H, Jenkinson E, Kabir S, Babul-Hirji R, Najm-Tehrani N, Chitayat DA, et al. A POT1 mutation implicates defective telomere end fill-in and telomere truncations in Coats plus. Genes Dev. 2016;30(7):812–26.
Kermasson L, Churikov D, Awad A, Smoom R, Lainey E, Touzot F, et al. Inherited human Apollo deficiency causes severe bone marrow failure and developmental defects. Blood. 2022;139(16):2427–40.
Polvi A, Linnankivi T, Kivelä T, Herva R, Keating James P, Mäkitie O, et al. Mutations in CTC1, encoding the CTS telomere maintenance complex component 1, cause cerebroretinal microangiopathy with calcifications and cysts. Am J Human Genet. 2012;90(3):540–9.
Simon AJ, Lev A, Zhang Y, Weiss B, Rylova A, Eyal E, et al. Mutations in STN1 cause coats plus syndrome and are associated with genomic and telomere defects. J Exp Med. 2016;213(8):1429–40.
Holme H, Hossain U, Kirwan M, Walne A, Vulliamy T, Dokal I. Marked genetic heterogeneity in familial myelodysplasia/acute myeloid leukaemia. Br J Haematol. 2012;158(2):242–8.
Furutani E, Shimamura A. Genetic predisposition to MDS: diagnosis and management. Hematol Am Soc Hematol Educ Program. 2019;2019(1):110–9.
Beerman I. Accumulation of DNA damage in the aged hematopoietic stem cell compartment. Semin Hematol. 2017;54(1):12–8.
Mollica L, Fleury I, Belisle C, Provost S, Roy DC, Busque L. No association between telomere length and blood cell counts in elderly individuals. J Gerontol A Biol Sci Med Sci. 2009;64(9):965–7.
Florian MC, Dörr K, Niebel A, Daria D, Schrezenmeier H, Rojewski M, et al. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem Cell. 2012;10(5):520–30.
Ho TT, Warr MR, Adelman ER, Lansinger OM, Flach J, Verovskaya EV, et al. Autophagy maintains the metabolism and function of young and old stem cells. Nature. 2017;543(7644):205–10.
Kramer A, Challen GA. The epigenetic basis of hematopoietic stem cell aging. Semin Hematol. 2017;54(1):19–24.
Papaemmanuil E, Gerstung M, Malcovati L, Tauro S, Gundem G, Van Loo P, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122(22):3616–27.
Walter MJ, Shen D, Shao J, Ding L, White BS, Kandoth C, et al. Clonal diversity of recurrently mutated genes in myelodysplastic syndromes. Leukemia. 2013;27(6):1275–82.
Kaner J, Desai P, Mencia-Trinchant N, Guzman ML, Roboz GJ, Hassane DC. Clonal Hematopoiesis and Premalignant Diseases. Cold Spring Harb Perspect Med. 2020;10(4):a035675.
Mitchell SR, Gopakumar J, Jaiswal S. Insights into clonal hematopoiesis and its relation to cancer risk. Curr Opin Genet Dev. 2021;66:63–9.
Belizaire R, Wong WJ, Robinette ML, Ebert BL. Clonal haematopoiesis and dysregulation of the immune system. Nat Rev Immunol. 2023;23(9):595–610.
Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–98.
Ferrer A, Lasho T, Fernandez JA, Steinauer NP, Simon RA, Finke CM, et al. Patients with telomere biology disorders show context specific somatic mosaic states with high frequency of U2AF1 variants. Am J Hematol. 2023;98(12):E357–9.
Revy P, Kannengiesser C, Fischer A. Somatic genetic rescue in Mendelian haematopoietic diseases. Nat Rev Genet. 2019;20(10):582–98.
Revy P, Kannengiesser C, Fischer A. Somatic genetic rescue in Mendelian haematopoietic diseases. Nat Rev Genet. 2019;20(10):582–98.
Tsai FD, Lindsley RC. Clonal hematopoiesis in the inherited bone marrow failure syndromes. Blood. 2020;136(14):1615–22.
Perdigones N, Perin JC, Schiano I, Nicholas P, Biegel JA, Mason PJ, et al. Clonal hematopoiesis in patients with dyskeratosis congenita. Am J Hematol. 2016;91(12):1227–33.
Mangaonkar AA, Ferrer A, Pinto EVF, Cousin MA, Kuisle RJ, Klee EW, et al. Clinical correlates and treatment outcomes for patients with short telomere syndromes. Mayo Clin Proc. 2018;93(7):834–9.
Lee M, Roos P, Sharma N, Atalar M, Evans TA, Pellicore MJ, et al. Systematic computational identification of variants that activate exonic and intronic cryptic splice sites. Am J Human Genet. 2017;100(5):751–65.
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell. 2023;186(2):243–78.
Marioni RE, Shah S, McRae AF, Chen BH, Colicino E, Harris SE, et al. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015;16(1):25.
Acknowledgements
We would like to acknowledge the Center for Individualized Medicine, Mayo Clinic, for establishing the telomere biology disorders clinic and biobank and all the patients for their participation.
Author information
Authors and Affiliations
Contributions
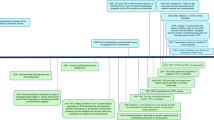
T.L. and M.P. wrote the main manuscript text and T.L. prepared figure 1.
Corresponding author
Ethics declarations
Ethics Approval
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki Declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Conflict of Interest
Dr. Lasho has nothing to disclose. Dr. Patnaik has received research funding from StemLine, Kura, Epigenetix, and Polaris and is currently on the advisory board for Center for Therapeutic Intervention (CTI).
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lasho, T., Patnaik, M.M. Adaptive and Maladaptive Clonal Hematopoiesis in Telomere Biology Disorders. Curr Hematol Malig Rep 19, 35–44 (2024). https://doi.org/10.1007/s11899-023-00719-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-023-00719-2