Abstract
Purpose of Review
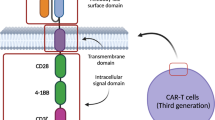
Chimeric antigen receptor (CAR) T cell therapy is an immunotherapy that has resulted in tremendous progress in the treatment of patients with B cell malignancies. However, the remarkable efficacy of therapy is not without significant safety concerns. Herein, we will review the unique and potentially life-threatening toxicities associated with CAR-T cell therapy and their association with treatment efficacy.
Recent Findings
Currently, CAR-T cell therapy is approved for the treatment of B cell relapsed or refractory leukemia and lymphoma, and most recently, multiple myeloma (MM). In these different diseases, it has led to excellent complete and overall response rates depending on the patient population and therapy. Despite promising efficacy, CAR-T cell therapy is associated with significant side effects; the two most notable toxicities are cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). The treatment of CAR-T-induced toxicity is supportive; however, as higher-grade adverse events occur, toxicity-directed therapy with tocilizumab, an IL-6 receptor antibody, and steroids is standard practice. Overall, a careful risk–benefit balance exists between the efficacy and toxicities of therapies. The challenge lies in the underlying pathophysiology of CAR-T-related toxicity which relies upon the activation of CAR-T cells.
Summary
Some degree of toxicity is expected to achieve an effective response to therapy, and certain aspects of treatment are also associated with toxicity. As progress is made in the investigation and approval of new CARs, novel toxicity-directed therapies and toxicity-limited constructs will be the focus of attention.

Similar content being viewed by others
Data Availability
This review article does not present any original unpublished data. All data presented is associated with the corresponding reference.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Almasbak H, Aarvak T, Vemuri MC. CAR T cell therapy: a game changer in cancer treatment. J Immunol Res. 2016;2016:5474602. https://doi.org/10.1155/2016/5474602.
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11:69. https://doi.org/10.1038/s41408-021-00459-7.
Guedan S, Calderon H, Posey AD Jr, Maus MV. Engineering and design of chimeric antigen receptors. Mol Ther Methods Clin Dev. 2019;12:145–56. https://doi.org/10.1016/j.omtm.2018.12.009.
Zhao J, Lin Q, Song Y, Liu D. Universal CARs, universal T cells, and universal CAR T cells. J Hematol Oncol. 2018;11:132. https://doi.org/10.1186/s13045-018-0677-2.
Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19:185–99. https://doi.org/10.1038/s41573-019-0051-2.
Brudno JN, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. 2016;34:1112–21. https://doi.org/10.1200/JCO.2015.64.5929.
Weinkove R, George P, Dasyam N, McLellan AD. Selecting costimulatory domains for chimeric antigen receptors: functional and clinical considerations. Clin Transl Immunology 8, e1049. https://doi.org/10.1002/cti2.1049 (2019).
June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–5. https://doi.org/10.1126/science.aar6711.
Finney HM, Lawson AD, Bebbington CR, Weir AN. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. 1998;161:2791–7.
Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20:70–5. https://doi.org/10.1038/nbt0102-70.
Zhao Y, et al. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J Immunol. 2009;183:5563–74. https://doi.org/10.4049/jimmunol.0900447.
••Fowler, N. H. et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med 28, 325-332. https://doi.org/10.1038/s41591-021-01622-0. Recent trial leading to the approval of Tisagenlecleucel for FL.
Schuster SJ, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. https://doi.org/10.1056/NEJMoa1804980.
Neelapu SS, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44. https://doi.org/10.1056/NEJMoa1707447.
Wang M, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331–42. https://doi.org/10.1056/NEJMoa1914347.
••Jacobson, C. A. et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol 23, 91-103. Recent clinical trial forming the basis of approval of axi-cel for the treatment of indolent lymphomas.
Berdeja JG, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398:314–24. https://doi.org/10.1016/S0140-6736(21)00933-8.
Abramson JS, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–52. https://doi.org/10.1016/S0140-6736(20)31366-0.
Maude SL, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48. https://doi.org/10.1056/NEJMoa1709866.
Shah BD, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398:491–502. https://doi.org/10.1016/S0140-6736(21)01222-8.
Munshi NC, et al. Idecabtagene Vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384:705–16. https://doi.org/10.1056/NEJMoa2024850.
Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. https://doi.org/10.1056/NEJMoa1103849.
••Lee, D. W. et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 25, 625-638. https://doi.org/10.1016/j.bbmt.2018.12.758. Guidelines by the American Society for Transplantation and Cellular Therapy (ASTCT) which have established a consensus grading system (Grades 1–4) for both CRS and ICANS.
Yakoub-Agha I, et al. Management of adults and children undergoing chimeric antigen receptor T-cell therapy: best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE). Haematologica. 2020;105:297–316. https://doi.org/10.3324/haematol.2019.229781.
Sotillo E, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5:1282–95. https://doi.org/10.1158/2159-8290.CD-15-1020.
Castellarin, M. et al. A rational mouse model to detect on-target, off-tumor CAR T cell toxicity. JCI Insight 5. https://doi.org/10.1172/jci.insight.136012 (2020).
Ghorashian S, et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat Med. 2019;25:1408–14. https://doi.org/10.1038/s41591-019-0549-5.
Majzner RG, Mackall CL. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018;8:1219–26. https://doi.org/10.1158/2159-8290.CD-18-0442.
Ahmadzadeh M, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–44. https://doi.org/10.1182/blood-2008-12-195792.
Baitsch L, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest. 2011;121:2350–60. https://doi.org/10.1172/JCI46102.
Long AH, et al. 4–1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21:581–90. https://doi.org/10.1038/nm.3838.
Gargett T, et al. GD2-specific CAR T cells undergo potent activation and deletion following antigen encounter but can be protected from activation-induced cell death by PD-1 blockade. Mol Ther. 2016;24:1135–49. https://doi.org/10.1038/mt.2016.63.
Heczey A, et al. CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol Ther. 2017;25:2214–24. https://doi.org/10.1016/j.ymthe.2017.05.012.
Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. https://doi.org/10.1038/nature04444.
Hay KA, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130:2295–306. https://doi.org/10.1182/blood-2017-06-793141.
Pennisi M, et al. Comparing CAR T-cell toxicity grading systems: application of the ASTCT grading system and implications for management. Blood Adv. 2020;4:676–86. https://doi.org/10.1182/bloodadvances.2019000952.
Neelapu SS, et al. Chimeric antigen receptor T-cell therapy — assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62. https://doi.org/10.1038/nrclinonc.2017.148.
Park JH, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–59. https://doi.org/10.1056/NEJMoa1709919.
Schuster SJ, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–54. https://doi.org/10.1056/NEJMoa1708566.
Murthy H, Iqbal M, Chavez JC, Kharfan-Dabaja MA. Cytokine release syndrome: current perspectives. Immunotargets Ther. 2019;8:43–52. https://doi.org/10.2147/ITT.S202015.
Sterner RM, et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood. 2019;133:697–709. https://doi.org/10.1182/blood-2018-10-881722.
Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20:119–22. https://doi.org/10.1097/PPO.0000000000000035.
Gabay C, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381:1541–50. https://doi.org/10.1016/S0140-6736(13)60250-0.
Bijlsma JWJ, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet. 2016;388:343–55. https://doi.org/10.1016/S0140-6736(16)30363-4.
Le RQ, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23:943–7. https://doi.org/10.1634/theoncologist.2018-0028.
Li M, et al. The differential effects of tumor burdens on predicting the net benefits of ssCART-19 cell treatment on r/r B-ALL patients. Sci Rep. 2022;12:378. https://doi.org/10.1038/s41598-021-04296-3.
Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17. https://doi.org/10.1056/NEJMoa1407222.
Teachey DT, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6:664–79. https://doi.org/10.1158/2159-8290.CD-16-0040.
Mahadeo KM, et al. Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy. Nat Rev Clin Oncol. 2019;16:45–63. https://doi.org/10.1038/s41571-018-0075-2.
Rubin DB, et al. Clinical predictors of neurotoxicity after chimeric antigen receptor T-cell therapy. JAMA Neurol. 2020;77:1536–42. https://doi.org/10.1001/jamaneurol.2020.2703.
Curran KJ, et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood. 2019;134:2361–8. https://doi.org/10.1182/blood.2019001641.
Santomasso BD, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8:958–71. https://doi.org/10.1158/2159-8290.CD-17-1319.
Gust J, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7:1404–19. https://doi.org/10.1158/2159-8290.CD-17-0698.
Grant SJ, et al. Clinical presentation, risk factors, and outcomes of immune effector cell-associated neurotoxicity syndrome following chimeric antigen receptor T cell therapy: a systematic review. Transplant Cell Ther. 2022;28:294–302. https://doi.org/10.1016/j.jtct.2022.03.006.
Schuster SJ, et al. Grading and management of cytokine release syndrome in patients treated with tisagenlecleucel in the JULIET trial. Blood Adv. 2020;4:1432–9. https://doi.org/10.1182/bloodadvances.2019001304.
Strati P, et al. Prognostic impact of corticosteroids on efficacy of chimeric antigen receptor T-cell therapy in large B-cell lymphoma. Blood. 2021;137:3272–6. https://doi.org/10.1182/blood.2020008865.
Nellan A, et al. Improved CNS exposure to tocilizumab after cerebrospinal fluid compared to intravenous administration in rhesus macaques. Blood. 2018;132:662–6. https://doi.org/10.1182/blood-2018-05-846428.
Balis FM, Lester CM, Chrousos GP, Heideman RL, Poplack DG. Differences in cerebrospinal fluid penetration of corticosteroids: possible relationship to the prevention of meningeal leukemia. J Clin Oncol. 1987;5:202–7. https://doi.org/10.1200/JCO.1987.5.2.202.
Labar B, et al. Dexamethasone compared to prednisolone for adults with acute lymphoblastic leukemia or lymphoblastic lymphoma: final results of the ALL-4 randomized, phase III trial of the EORTC Leukemia Group. Haematologica. 2010;95:1489–95. https://doi.org/10.3324/haematol.2009.018580.
•Gardner, R. A. et al. Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy. Blood 134, 2149-2158. https://doi.org/10.1182/blood.2019001463. Study which reported that early intervention with steroids or tocilizumab may reduce the potential development for severe toxicity and have minimal impact on treatment efficacy.
Chen F, et al. Measuring IL-6 and sIL-6R in serum from patients treated with tocilizumab and/or siltuximab following CAR T cell therapy. J Immunol Methods. 2016;434:1–8. https://doi.org/10.1016/j.jim.2016.03.005.
Titov A, et al. The biological basis and clinical symptoms of CAR-T therapy-associated toxicites. Cell Death Dis. 2018;9:897. https://doi.org/10.1038/s41419-018-0918-x.
Zhang L, et al. Etanercept as a new therapeutic option for cytokine release syndrome following chimeric antigen receptor T cell therapy. Exp Hematol Oncol. 2021;10:16. https://doi.org/10.1186/s40164-021-00209-2.
Strati P, et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy-associated toxicity in large B-cell lymphoma. Blood Adv. 2020;4:3123–7. https://doi.org/10.1182/bloodadvances.2020002328.
Shi Y, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res. 2006;16:126–33. https://doi.org/10.1038/sj.cr.7310017.
Yi Y, et al. CRISPR-edited CART with GM-CSF knockout and auto secretion of IL6 and IL1 blockers in patients with hematologic malignancy. Cell Discov. 2021;7:27. https://doi.org/10.1038/s41421-021-00255-4.
Kenderian SS, et al. ZUMA-19: A phase 1/2 multicenter study of lenzilumab use with axicabtagene ciloleucel (Axi-Cel) in patients (Pts) with relapsed or refractory large B cell lymphoma (R/R LBCL). Blood. 2020;136:6–7. https://doi.org/10.1182/blood-2020-135988.
Wang K, Wei G, Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol. 2012;1:36. https://doi.org/10.1186/2162-3619-1-36.
Engel P, et al. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity. 1995;3:39–50. https://doi.org/10.1016/1074-7613(95)90157-4.
Maude SL, et al. Sustained remissions with CD19-specific chimeric antigen receptor (CAR)-modified T cells in children with relapsed/refractory ALL. J Clin Oncol. 2016;34:3011–3011. https://doi.org/10.1200/JCO.2016.34.15_suppl.3011.
Anagnostou T, Riaz IB, Hashmi SK, Murad MH, Kenderian SS. Anti-CD19 chimeric antigen receptor T-cell therapy in acute lymphocytic leukaemia: a systematic review and meta-analysis. Lancet Haematol. 2020;7:e816–26. https://doi.org/10.1016/S2352-3026(20)30277-5.
Locke FL, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20:31–42. https://doi.org/10.1016/S1470-2045(18)30864-7.
Nastoupil LJ, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T consortium. J Clin Oncol. 2020;38:3119–28. https://doi.org/10.1200/JCO.19.02104.
Jacobson CA, et al. Axicabtagene ciloleucel in the non-trial setting: outcomes and correlates of response, resistance, and toxicity. J Clin Oncol. 2020;38:3095–106. https://doi.org/10.1200/JCO.19.02103.
Pasquini MC, et al. Post-marketing use outcomes of an anti-CD19 chimeric antigen receptor (CAR) T cell therapy, Axicabtagene ciloleucel (axi-cel), for the treatment of large B cell lymphoma (LBCL) in the United States (US). Blood. 2019;134:764–764. https://doi.org/10.1182/blood-2019-124750.
Locke FL, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386:640–54. https://doi.org/10.1056/NEJMoa2116133.
Bishop MR, et al. Second-line Tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2022;386:629–39. https://doi.org/10.1056/NEJMoa2116596.
Kamdar M, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399:2294–308. https://doi.org/10.1016/S0140-6736(22)00662-6.
Perales, M. A. et al. Role of CD19 chimeric antigen receptor T cells in second-line large B cell lymphoma: lessons from phase 3 trials. An Expert Panel Opinion from the American Society for Transplantation and Cellular Therapy. Transplant Cell Ther 28, 546–559. https://doi.org/10.1016/j.jtct.2022.06.019 (2022).
•Iacoboni, G. et al. Real-world evidence of brexucabtagene autoleucel for the treatment of relapsed or refractory mantle cell lymphoma. Blood Adv. https://doi.org/10.1182/bloodadvances.2021006922. Recently published real-world analysis of brexi-cel in patients with MCL which serves to validate clinical trial data from ZUMA-2.
Shah N, Chari A, Scott E, Mezzi K, Usmani SZ. B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches. Leukemia. 2020;34:985–1005. https://doi.org/10.1038/s41375-020-0734-z.
Sanchez E, et al. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. Br J Haematol. 2012;158:727–38. https://doi.org/10.1111/j.1365-2141.2012.09241.x.
Ghilardi G, et al. Bendamustine is safe and effective for lymphodepletion before tisagenlecleucel in patients with refractory or relapsed large B-cell lymphomas. Ann Oncol. 2022;33:916–28. https://doi.org/10.1016/j.annonc.2022.05.521.
Yoon, D. H., Osborn, M. J., Tolar, J. & Kim, C. J. Incorporation of immune checkpoint blockade into chimeric antigen receptor T cells (CAR-Ts): combination or built-in CAR-T. Int J Mol Sci 19. https://doi.org/10.3390/ijms19020340 (2018).
Ceschi A, Noseda R, Palin K, Verhamme K. Immune checkpoint inhibitor-related cytokine release syndrome: analysis of WHO global pharmacovigilance database. Front Pharmacol. 2020;11:557. https://doi.org/10.3389/fphar.2020.00557.
Yeku, O. O., Purdon, T., Spriggs, D. R. & Brentjens, R. J. Chimeric antigen receptor (CAR) T cells genetically engineered to deliver IL-12 to the tumor microenvironment in ovarian cancer. Journal of Clinical Oncology 35.https://doi.org/10.1200/Jco.2017.35.15_Suppl.3050 (2017).
Yeku, O. O., Purdon, T., Spriggs, D. R. & Brentjens, R. J. Interleukin-12 armored chimeric antigen receptor (CAR) T cells for heterogeneous antigen-expressing ovarian cancer. Journal of Clinical Oncology 36. https://doi.org/10.1200/Jco.2018.36.5_Suppl.12 (2018).
Koneru, M., Purdon, T. J., Spriggs, D., Koneru, S. & Brentjens, R. J. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology 4, doi:ARTN e994446 https://doi.org/10.4161/2162402X.2014.994446 (2015).
Choi BD, et al. CRISPR-Cas9 disruption of PD-1 enhances activity of universal EGFRvIII CAR T cells in a preclinical model of human glioblastoma. J Immunother Cancer. 2019;7:304. https://doi.org/10.1186/s40425-019-0806-7.
Hoyos V, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24:1160–70. https://doi.org/10.1038/leu.2010.75.
Budde, L. E. et al. Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma. PLoS One 8, e82742. https://doi.org/10.1371/journal.pone.0082742 (2013).
Wang X, et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118:1255–63. https://doi.org/10.1182/blood-2011-02-337360.
Tasian, S. K. et al. Optimized depletion of chimeric antigen receptor T-cells in murine xenograft models of human acute myeloid leukemia. Blood. https://doi.org/10.1182/blood-2016-08-736041
Diaconu I, et al. Inducible caspase-9 selectively modulates the toxicities of CD19-specific chimeric antigen receptor-modified T cells. Mol Ther. 2017;25:580–92. https://doi.org/10.1016/j.ymthe.2017.01.011.
Stroncek DF, et al. Myeloid cells in peripheral blood mononuclear cell concentrates inhibit the expansion of chimeric antigen receptor T cells. Cytotherapy. 2016;18:893–901. https://doi.org/10.1016/j.jcyt.2016.04.003.
Ruella M, et al. Overcoming the immunosuppressive tumor microenvironment of Hodgkin lymphoma using chimeric antigen receptor T cells. Cancer Discov. 2017;7:1154–67. https://doi.org/10.1158/2159-8290.CD-16-0850.
Giavridis T, et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24:731–8. https://doi.org/10.1038/s41591-018-0041-7.
Teachey DT, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6:664–79. https://doi.org/10.1158/2159-8290.CD-16-0040.
Gauthier J, et al. Feasibility and efficacy of CD19-targeted CAR-T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood. 2020;135:1650–60. https://doi.org/10.1182/blood.2019002936.
Author information
Authors and Affiliations
Contributions
K. L. C, S. S.K; wrote the initial manuscript: E. L. S contributed to the writing and editing of the final manuscript. All authors approved the submitted version.
Corresponding author
Ethics declarations
Conflict of Interest
SSK is inventor on patents in the CART cell field that are licensed to Novartis (through an agreement between Mayo Clinic, University of Pennsylvania, and Novartis), Humanigen (through Mayo Clinic), Mettaforge (through Mayo Clinic), MustangBio (through Mayo Clinic), and Sendero (through Mayo Clinic). SSK has received funding from Novartis, Kite/Gilead, Juno/BMS, Lentigen, Humanigen, Tolero, Morphosys, Sunesis, and Viracta, and LeahLabs. SSK has participated in scientific advisory board meetings with Kite/Gilead, Novartis, BMS, CapstanBio, Luminary therapeutics, and Humanigen. SSK has participated in DSMB meetings with Humanigen. SSK has participated in consulting activities with Novartis, Torque, CapstanBio, Luminary therapeutics, and Calibr. SSK hold equity in Life Engine, Inc. and Leahlabs. KLC and ELS have no conflicts to report.
Human and Animal Rights and Informed Consent.
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on CART and Immunotherapy.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chohan, K.L., Siegler, E.L. & Kenderian, S.S. CAR-T Cell Therapy: the Efficacy and Toxicity Balance. Curr Hematol Malig Rep 18, 9–18 (2023). https://doi.org/10.1007/s11899-023-00687-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-023-00687-7



