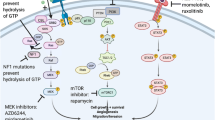
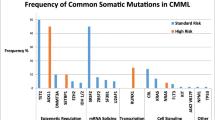
Abstract
Purpose of review
For decades, the management of chronic myelomonocytic leukemia (CMML) or juvenile myelomonocytic leukemia (JMML) has been largely inextricable from myelodysplastic syndromes (MDS), myeloproliferative neoplasms, and acute myeloid leukemia. Hallmarks of these diseases have been the emergence of unique genomic signatures and discouraging responses to available therapies. Here, we will critically examine the current options for management and review the rapidly developing opportunities based on advances in CMML and JMML disease biology.
Recent findings
Few clinical trials have exclusively been done in CMML, and in JMML, the rarity of the disease limits wide scale participation. Recent case series in JMML suggest that hypomethylating agents (HMAs) are a viable option for bridging to curative intent with allogeneic hematopoietic stem cell transplant or as posttransplant maintenance. Emerging evidence has demonstrated targeting the RAS-pathway via MEK inhibition may also be considered. In CMML, treatment with HMAs is largely derived from data inclusive of MDS patients, including a small number of patients with dysplastic CMML variants. Based on CMML disease biology, additional therapeutic targets being investigated include inhibitors of splicing, CD123/dendritic cell axis, inherent GM-CSF progenitor cell hypersensitivity, and targeting the JAK/STAT pathway. Current evidence is also expanding for oral HMAs.
Summary
The management of CMML and JMML is rapidly evolving and clinicians must be aware of the genetic landscape and expanding treatment options to ensure these rare populations are afforded therapeutic interventions best suited to their needs.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–405.
Patnaik MM, Itzykson R, Lasho TL, Kosmider O, Finke CM, Hanson CA, et al. ASXL1 and SETBP1 mutations and their prognostic contribution in chronic myelomonocytic leukemia: a two-center study of 466 patients. Leukemia. 2014;28(11):2206–12.
Patnaik MM, Tefferi A. Chronic Myelomonocytic leukemia: 2020 update on diagnosis, risk stratification and management. Am J Hematol. 2020;95(1):97–115.
Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–25.
Coston T, Pophali P, Vallapureddy R, Lasho TL, Finke CM, Ketterling RP, et al. Suboptimal response rates to hypomethylating agent therapy in chronic myelomonocytic leukemia; a single institutional study of 121 patients. Am J Hematol. 2019;94(7):767–79 Large cohort demonstrating poor response to hypomethylating agents in CMML.
Duchmann M, Braun T, Micol JB, Platzbecker U, Park S, Pilorge S, et al. Validation of response assessment according to international consortium for MDS/MPN criteria in chronic myelomonocytic leukemia treated with hypomethylating agents. Blood Cancer J. 2017;7(5):e562.
Savona MR, Malcovati L, Komrokji R, Tiu RV, Mughal TI, Orazi A, et al. An international consortium proposal of uniform response criteria for myelodysplastic/myeloproliferative neoplasms (MDS/MPN) in adults. Blood. 2015;125(12):1857–65.
Grossmann V, Kohlmann A, Eder C, Haferlach C, Kern W, Cross NC, et al. Molecular profiling of chronic myelomonocytic leukemia reveals diverse mutations in >80% of patients with TET2 and EZH2 being of high prognostic relevance. Leukemia. 2011;25(5):877–9.
Coltro G, Mangaonkar AA, Lasho TL, Finke CM, Pophali P, Carr R, et al. Clinical, molecular, and prognostic correlates of number, type, and functional localization of TET2 mutations in chronic myelomonocytic leukemia (CMML)-a study of 1084 patients. Leukemia. 2020;34(5):1407–21.
Itzykson R, Kosmider O, Renneville A, Gelsi-Boyer V, Meggendorfer M, Morabito M, et al. Prognostic score including gene mutations in chronic myelomonocytic leukemia. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31(19):2428–36.
Elena C, Gallì A, Such E, Meggendorfer M, Germing U, Rizzo E, et al. Integrating clinical features and genetic lesions in the risk assessment of patients with chronic myelomonocytic leukemia. Blood. 2016;128(10):1408–17.
Buradkar A, Bezerra E, Coltro G, Lasho TL, Finke CM, Gangat N, et al. Landscape of RAS pathway mutations in patients with myelodysplastic syndrome/myeloproliferative neoplasm overlap syndromes: a study of 461 molecularly annotated patients. Leukemia. 2021;35(2):644–9.
Kantarjian H, Issa J-PJ, Rosenfeld CS, Bennett JM, Albitar M, Dipersio J, et al. Decitabine improves patient outcomes in myelodysplastic syndromes. Cancer. 2006;106(8):1794–803.
Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, et al. Randomized Controlled Trial of Azacitidine in Patients With the Myelodysplastic Syndrome: A Study of the Cancer and Leukemia Group B. J Clin Oncol. 2002;20(10):2429–40.
Guerra V, Ramos Perez J, Hammond D, Chien K, Kanagal-Shamanna R, Naqvi K, et al. Outcomes of Chronic Myelomonocytic Leukemia (CMML) after Hypomethylating Agent (HMA) Failure. Blood. 2020;136(Supplement 1):22–3.
Ramos Perez J, Guerra V, Hammond D, Chien K, Kanagal-Shamanna R, Naqvi K, et al. Response and Survival Outcomes with Hypomethylating Agents in Patients with Chronic Myelomonocytic Leukemia Based on Disease Phenotype and Risk Categories. Blood. 2020;136:8–9.
Itzykson R, Santini V, Chaffaut C, Lionel A, Thepot S, Giagounidis A, et al. Decitabine Versus Hydroxyurea for Advanced Proliferative CMML: Results of the Emsco Randomized Phase 3 Dacota Trial. Blood. 2020;136(Supplement 1):53–4 Prospective, phase 3 trial demonstrating no difference in efficacy for decitabine versus hydroxyurea in advanced proliferative CMML.
Merlevede J, Droin N, Qin T, Meldi K, Yoshida K, Morabito M, et al. Mutation allele burden remains unchanged in chronic myelomonocytic leukaemia responding to hypomethylating agents. Nat Commun. 2016;7(1):10767.
Camiener GW, Smith CG. Studies of the enzymatic deamination of cytosine arabinoside—I: Enzyme distribution and species specificity. Biochem Pharmacol. 1965;14(10):1405–16.
Garcia-Manero G, McCloskey J, Griffiths E, Yee K, Zeidan A, Al-Kali A, et al. Pharmacokinetic Exposure Equivalence and Preliminary Efficacy and Safety from a Randomized Cross over Phase 3 Study (ASCERTAIN study) of an Oral Hypomethylating Agent ASTX727 (cedazuridine/decitabine) Compared to IV Decitabine. Blood. 2019;134(Supplement 1):846.
Garcia-Manero G, Gore SD, Cogle C, Ward R, Shi T, Macbeth KJ, et al. Phase I study of oral azacitidine in myelodysplastic syndromes, chronic myelomonocytic leukemia, and acute myeloid leukemia. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(18):2521–7.
Garcia-Manero G, Gore SD, Kambhampati S, Scott B, Tefferi A, Cogle CR, et al. Efficacy and safety of extended dosing schedules of CC-486 (oral azacitidine) in patients with lower-risk myelodysplastic syndromes. Leukemia. 2016;30(4):889–96.
Garcia-Manero G, Stoltz ML, Ward MR, Kantarjian H, Sharma S. A pilot pharmacokinetic study of oral azacitidine. Leukemia. 2008;22(9):1680–4.
Savona MR, Kolibaba K, Conkling P, Kingsley EC, Becerra C, Morris JC, et al. Extended dosing with CC-486 (oral azacitidine) in patients with myeloid malignancies. Am J Hematol. 2018;93(10):1199–206.
Hammond D, DiNardo C, Konopleva M, Borthakur G, Ramos Perez J, Guerra V. Activity of Venetoclax-Based Therapy in CMML and CMML with Blast Transformation. Blood. 2020;136(Supplement 1):36–7.
Morita K, Naqvi K, Montalban Bravo G, Thompson P, Takahashi K, Alvarado Y, et al. Initial Results of a Phase I/II Study of Venetoclax in Combination with Azacitidine in Treatment-Naive and Relapsed/Refractory High-Risk Myelodysplastic Syndrome (MDS) or Chronic Myelomonocytic Leukemia (CMML). Blood. 2020;136(Supplement 1):39–40.
Pei S, Pollyea DA, Gustafson A, Stevens BM, Minhajuddin M, Fu R, et al. Monocytic Subclones Confer Resistance to Venetoclax-Based Therapy in Patients with Acute Myeloid Leukemia. Cancer Discov. 2020;10(4):536–51 Demonstration of highly differentiated monocytic leukemias may be inherently refractory to venetoclax, a highly useful medication in current treatment algorithms.
Padron E, Dezern A, Andrade-Campos M, Vaddi K, Scherle P, Zhang Q, et al. A Multi-Institution Phase I Trial of Ruxolitinib in Patients with Chronic Myelomonocytic Leukemia (CMML). Clin Cancer Res. 2016;22(15):3746–54.
Assi R, Kantarjian HM, Garcia-Manero G, Cortes JE, Pemmaraju N, Wang X, et al. A phase II trial of ruxolitinib in combination with azacytidine in myelodysplastic syndrome/myeloproliferative neoplasms. Am J Hematol. 2018;93(2):277–85.
Ma Y, Rix L, Zhang Q, Balasis ME, Komrokji RS, Rix U, et al. Pacritinib (PAC) Synergistically Potentiates Azacitidine (5AZA) Cytotoxicity in Chronic Myelomonocytic Leukemia (CMML). Blood. 2015;126(23):1658.
Mesa RA, Kiladjian J-J, Catalano JV, Devos T, Egyed M, Hellmann A, et al. SIMPLIFY-1: A Phase III Randomized Trial of Momelotinib Versus Ruxolitinib in Janus Kinase Inhibitor-Naïve Patients With Myelofibrosis. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(34):3844–50.
Komrokji RS, Wei S, Mailloux AW, Zhang L, Padron E, Sallman D, et al. A Phase II Study to Determine the Safety and Efficacy of the Oral Inhibitor of Indoleamine 2,3-Dioxygenase (IDO) Enzyme INCB024360 in Patients with Myelodysplastic Syndromes. Clin Lymphoma Myeloma Leuk. 2019;19(3):157–61.
Issa J-PJ, Roboz G, Rizzieri D, Jabbour E, Stock W, O'Connell C, et al. Safety and tolerability of guadecitabine (SGI-110) in patients with myelodysplastic syndrome and acute myeloid leukaemia: a multicentre, randomised, dose-escalation phase 1 study. Lancet Oncol. 2015;16(9):1099–110.
Garcia-Manero G, Roboz G, Walsh K, Kantarjian H, Ritchie E, Kropf P, et al. Guadecitabine (SGI-110) in patients with intermediate or high-risk myelodysplastic syndromes: phase 2 results from a multicentre, open-label, randomised, phase 1/2 trial. Lancet Haematol. 2019;6(6):e317–e27.
Lucas N, Duchmann M, Rameau P, Noël F, Michea P, Saada V, et al. Biology and prognostic impact of clonal plasmacytoid dendritic cells in chronic myelomonocytic leukemia. Leukemia. 2019;33(10):2466–80.
Zahid MF, Barraco D, Lasho TL, Finke C, Ketterling RP, Gangat N, et al. Spectrum of autoimmune diseases and systemic inflammatory syndromes in patients with chronic myelomonocytic leukemia. Leuk Lymphoma. 2017;58(6):1488–93.
Le Naour J, Galluzzi L, Zitvogel L, Kroemer G, Vacchelli E. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology. 2020;9(1):1777625.
Mangaonkar AA, Reichard KK, Binder M, Coltro G, Lasho TL, Carr RM, et al. Bone marrow dendritic cell aggregates associate with systemic immune dysregulation in chronic myelomonocytic leukemia. Blood Adv. 2020;4(21):5425–30.
Mangaonkar A, Mondal AK, Fulzule S, Pundkar C, Park EJ, Jillella A, et al. A novel immunohistochemical score to predict early mortality in acute myeloid leukemia patients based on indoleamine 2,3 dioxygenase expression. Sci Rep. 2017;7(1):12892.
Padron E, Painter JS, Kunigal S, Mailloux AW, McGraw K, McDaniel JM, et al. GM-CSF-dependent pSTAT5 sensitivity is a feature with therapeutic potential in chronic myelomonocytic leukemia. Blood. 2013;121(25):5068–77.
Patnaik MM, Sallman DA, Mangaonkar AA, Heuer R, Hirvela J, Zblewski D, et al. Phase 1 study of lenzilumab, a recombinant anti–human GM-CSF antibody, for chronic myelomonocytic leukemia. Blood. 2020;136(7):909–13.
Patnaik MM, Lasho TL, Finke CM, Hanson CA, Hodnefield JM, Knudson RA, et al. Spliceosome mutations involving SRSF2, SF3B1, and U2AF35 in chronic myelomonocytic leukemia: Prevalence, clinical correlates, and prognostic relevance. Am J Hematol. 2013;88(3):201–6.
Wudhikarn K, Loghavi S, Mangaonkar AA, Al-Kali A, Binder M, Carr R, et al. SF3B1-mutant CMML defines a predominantly dysplastic CMML subtype with a superior acute leukemia-free survival. Blood Adv. 2020;4(22):5716–21.
Steensma DP, Wermke M, Klimek V, Greenberg PL, Font P, Komrokji R, et al. Results of a Clinical Trial of H3B-8800, a Splicing Modulator, in Patients with Myelodysplastic Syndromes (MDS), Acute Myeloid Leukemia (AML) or Chronic Myelomonocytic Leukemia (CMML). Blood. 2019;134(Supplement 1):673.
Eskens FALM, Ramos FJ, Burger H, O'Brien JP, Piera A, de Jonge MJA, et al. Phase I Pharmacokinetic and Pharmacodynamic Study of the First-in-Class Spliceosome Inhibitor E7107 in Patients with Advanced Solid Tumors. Clin Cancer Res. 2013;19(22):6296–304.
Carr RM, Vorobyev D, Lasho T, Marks DL, Tolosa EJ, Vedder A, et al. RAS mutations drive proliferative chronic myelomonocytic leukemia via activation of a novel KMT2A-PLK1 axis. bioRxiv. 2019;12(23):874487.
Cortes J, Podoltsev N, Kantarjian H, Borthakur G, Zeidan AM, Stahl M, et al. Phase 1 dose escalation trial of volasertib in combination with decitabine in patients with acute myeloid leukemia. Int J Hematol. 2021;113(1):92–9.
Zeidan AM, Ridinger M, Lin TL, Becker PS, Schiller GJ, Patel PA, et al. A Phase Ib Study of Onvansertib, a Novel Oral PLK1 Inhibitor, in Combination Therapy for Patients with Relapsed or Refractory Acute Myeloid Leukemia. Clin Cancer Res. 2020;26(23):6132–40.
Watts J, Adès L, Radinoff A, Sangerman MA, Cerrano M, Lopez PF, et al. MDS-336: Phase 2 Study of Pevonedistat + Azacitidine versus Azacitidine in Patients with Higher-Risk Myelodysplastic Syndromes (MDS)/Chronic Myelomonocytic Leukemia (CMML) or Low-Blast Acute Myelogenous Leukemia (LB-AML) (NCT02610777): Subset Analysis in Higher-Risk MDS. Clin Lymphoma Myeloma Leuk. 2020;20:S323–S4.
Locatelli F, Algeri M, Merli P, Strocchio L. Novel approaches to diagnosis and treatment of Juvenile Myelomonocytic Leukemia. Expert Rev Hematol. 2018;11(2):129–43.
Patnaik MM, Lasho T. Myelodysplastic syndrome/myeloproliferative neoplasm overlap syndromes: a focused review. Hematol Am Soc Hematol Educ Program. 2020;2020(1):460–4.
Stieglitz E, Taylor-Weiner AN, Chang TY, Gelston LC, Wang YD, Mazor T, et al. The genomic landscape of juvenile myelomonocytic leukemia. Nat Genet. 2015;47(11):1326–33.
Mutz ID, Humphrey GB, Henderson ES. “Chronic myelogenous” leukemia of juvenile type. Report of two cases and review of therapy. Eur J Pediatr. 1976;121(4):227–36.
Bernard J, Seligmann M, Acar J. Chronic myeloid leukemia in the child (study of 20 cases). Arch Fr Pediatr. 1962;19:881–94.
Matsuda K, Shimada A, Yoshida N, Ogawa A, Watanabe A, Yajima S, et al. Spontaneous improvement of hematologic abnormalities in patients having juvenile myelomonocytic leukemia with specific RAS mutations. Blood. 2007;109(12):5477–80.
Wajid MA, Gupta AK, Das G, Sahoo D, Meena JP, Seth R. Outcomes of juvenile myelomonocytic leukemia patients after sequential therapy with cytarabine and 6-mercaptopurine. Pediatr Hematol Oncol. 2020;37(7):573–81.
Bergstraesser E, Hasle H, Rogge T, Fischer A, Zimmermann M, Noellke P, et al. Non-hematopoietic stem cell transplantation treatment of juvenile myelomonocytic leukemia: a retrospective analysis and definition of response criteria. Pediatr Blood Cancer. 2007;49(5):629–33.
Locatelli F, Nollke P, Zecca M, Korthof E, Lanino E, Peters C, et al. Hematopoietic stem cell transplantation (HSCT) in children with juvenile myelomonocytic leukemia (JMML): results of the EWOG-MDS/EBMT trial. Blood. 2005;105(1):410–9.
Locatelli F, Niemeyer CM. How I treat juvenile myelomonocytic leukemia. Blood. 2015;125(7):1083–90.
Dvorak CC, Satwani P, Stieglitz E, Cairo MS, Dang H, Pei Q, et al. Disease burden and conditioning regimens in ASCT1221, a randomized phase II trial in children with juvenile myelomonocytic leukemia: A Children's Oncology Group study. Pediatr Blood Cancer. 2018;65(7):e27034.
Flotho C, Sommer S, Lubbert M. DNA-hypomethylating agents as epigenetic therapy before and after allogeneic hematopoietic stem cell transplantation in myelodysplastic syndromes and juvenile myelomonocytic leukemia. Semin Cancer Biol. 2018;51:68–79.
Niemeyer CM, Flotho C, Lipka DB, Starý J, Rössig C, Baruchel A, et al. Upfront azacitidine (AZA) in juvenile myelomonocytic leukemia (JMML): Interim analysis of the prospective AZA-JMML-001 study. J Clin Oncol. 2019;37(15_suppl):10031 Interim analysis of the first prospective, phase 2 multicenter trial of hypomethylating agents for pediatric patients with JMML.
Cseh A, Niemeyer CM, Yoshimi A, Dworzak M, Hasle H, van den Heuvel-Eibrink MM, et al. Bridging to transplant with azacitidine in juvenile myelomonocytic leukemia: a retrospective analysis of the EWOG-MDS study group. Blood. 2015;125(14):2311–3.
Hecht A, Meyer J, Chehab FF, White KL, Magruder K, Dvorak CC, et al. Molecular assessment of pretransplant chemotherapy in the treatment of juvenile myelomonocytic leukemia. Pediatr Blood Cancer. 2019;66(11):e27948 Single-center study establishing the importance of monitoring for and achieving molecular response pre-HSCT for JMML.
Furlan I, Batz C, Flotho C, Mohr B, Lubbert M, Suttorp M, et al. Intriguing response to azacitidine in a patient with juvenile myelomonocytic leukemia and monosomy 7. Blood. 2009;113(12):2867–8.
Hashmi SK, Punia JN, Marcogliese AN, Gaikwad AS, Fisher KE, Roy A, et al. Sustained remission with azacitidine monotherapy and an aberrant precursor B-lymphoblast population in juvenile myelomonocytic leukemia. Pediatr Blood Cancer. 2019;66(10):e27905.
Fabri O, Horakova J, Bodova I, Svec P, Laluhova Striezencova Z, Bubanska E, Cermak M, Galisova V, Skalicka K, Vaska A, Doczyova D, Panikova A, Sykora T, Adamcakova J, Kolenova A. Diagnosis and treatment of juvenile myelomonocytic leukemia in Slovak Republic: novel approaches. Neoplasma. 2019. 181231N1009. https://pubmed.ncbi.nlm.nih.gov/31088105/.
Marcu A, Colita A, Radu LE, Jercan CG, Bica AM, Asan M, et al. Single-Center Experience With Epigenetic Treatment for Juvenile Myelomonocytic Leukemia. Front Oncol. 2020;10:484.
Ai Y, Lu X, Zhu T, Zhu Y, Liu H, Sun S. Combination of DNA-hypomethylating agent and hematopoietic stem cell transplantation in treatment of juvenile myelomonocytic leukemia: A case report. Medicine (Baltimore). 2020;99(50):e23606.
Peng Z, Feng X, He Y, Lin Y, Liao J, Wu X, et al. Hypomethylation of Decitabine Improved Outcomes of Hematopoietic Stem Cell Transplantation in Children with Juvenile Myelomonocytic Leukemia. Blood. 2017;130(Supplement 1):3232.
Peng Z, Feng X, Liu H, He Y, Liao J, Liu X, et al. A Novel Therapy Protocol Improves Outcomes of Hematopoietic Stem Cell Transplantation in Juvenile Myelomonocytic Leukemia Patients Using Hypomethylation of Decitabine. Blood. 2018;132(Supplement 1):5778.
Oshrine BR, Shyr D, Hale G, Petrovic A. Low-dose azacitidine for relapse prevention after allogeneic hematopoietic cell transplantation in children with myeloid malignancies. Pediatr Transplant. 2019;23(4):e13423.
Tamura A, Ishida T, Saito A, Yamamoto N, Yokoi T, Uemura S, et al. Low-dose azacitidine maintenance therapy after allogeneic stem cell transplantation for high-risk pediatric acute myeloid leukemia. Pediatr Blood Cancer. 2018;65(10):e27284.
Wahlstrom JT, Horn BN, Fraser-Browne C, Hoeweler R, Lu Y, Melton A, et al. Azacitidine Administration Following Hematopoietic Stem Cell Transplantation Is Safe and Feasible in Children with Acute Leukemia. Blood. 2016;128(22):4805.
Schonung M, Meyer J, Nollke P, Olshen AB, Hartmann M, Murakami N, et al. International Consensus Definition of DNA Methylation Subgroups in Juvenile Myelomonocytic Leukemia. Clin Cancer Res. 2021;27(1):158–68 Meta-analysis of large JMML cohort establishing discrete DNA methylation subgroups to be used in risk-adapted clinical trial design.
Cseh AM, Niemeyer CM, Yoshimi A, Catala A, Fruhwald MC, Hasle H, et al. Therapy with low-dose azacitidine for MDS in children and young adults: a retrospective analysis of the EWOG-MDS study group. Br J Haematol. 2016;172(6):930–6.
Meynier S, Rieux-Laucat F. After 95 years, it's time to eRASe JMML. Blood Rev. 2020;43:100652.
Tasian SK, Casas JA, Posocco D, Gandre-Babbe S, Gagne AL, Liang G, et al. Mutation-specific signaling profiles and kinase inhibitor sensitivities of juvenile myelomonocytic leukemia revealed by induced pluripotent stem cells. Leukemia. 2019;33(1):181–90.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kristen McCullough and Alexis Kuhn declare that they has no conflicts of interest. Mrinal Patnaik has served on the advisory boards for Stem Line Pharmaceuticals and Kura Oncology
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topical Collection on Myelodysplastic Syndromes and MPN/MDS Overlap
Rights and permissions
About this article
Cite this article
McCullough, K.B., Kuhn, A.K. & Patnaik, M.M. Treatment advances for pediatric and adult onset neoplasms with monocytosis. Curr Hematol Malig Rep 16, 256–266 (2021). https://doi.org/10.1007/s11899-021-00622-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-021-00622-8