Abstract
Purpose of Review
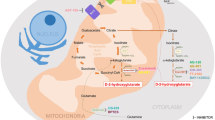
Mutations in isocitrate dehydrogenase genes (IDH1 and IDH2) are common in acute myeloid leukemia (AML), occurring in up to 30% of AML cases. Mutations in IDH leads to abnormal epigenetic regulation in AML cells and blocks differentiation. Inhibitors of mutated IDH1 and IDH2, ivosidenib and enasidenib, respectively, were recently approved by the FDA for relapsed/refractory AML; ivosidenib is also approved for newly diagnosed AML patients not fit for standard chemotherapy. Here, we discuss the clinical development of IDH inhibitors, their unique side effects, and outline future combination approaches in AML.
Recent Findings
IDH inhibitors are well-tolerated but can induce differentiation of AML cells, which leads to the on-target side effect of differentiation syndrome in up to 20% of patients. Although IDH inhibitors demonstrate efficacy as monotherapy, recent trials have shown that they have higher response rates in combination with hypomethylating agents (HMAs). Current trials of IDH inhibitors include combination with standard induction chemotherapy, as maintenance therapy, and in combination with venetoclax-based regimens.
Summary
IDH inhibitors are active and have a favorable toxicity profile in AML therapy. Current clinical trials are evaluating how to best incorporate IDH inhibitors into combination therapy to optimize outcomes and duration of response for AML patients with IDH mutations.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209–21. Comprehensive paper defining genetic abnormalities in AML.
Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–47.
Gilliland DG. Molecular genetics of human leukemias: new insights into therapy. Semin Hematol. 2002;39(4 Suppl 3):6–11.
Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003;3(9):650–65.
Fletcher L, Joshi SK, Traer E. Profile of quizartinib for the treatment of adult patients with relapsed/refractory FLT3-ITD-positive acute myeloid leukemia: evidence to date. Cancer Manag Res. 2020;12(1179-1322 (Print)):151–63.
Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111–21.
Cancer Genome Atlas Research Network, Fau LT, Miller C, Fau MC, Ding L, Fau DL, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059–74. First genomic report of AML genetic abnormalities.
Abdel-Wahab O, Levine RL. Mutations in epigenetic modifiers in the pathogenesis and therapy of acute myeloid leukemia. Blood. 2013;121(18):3563–72.
Figueroa ME, Skrabanek L, Li Y, Jiemjit A, Fandy TE, Paietta E, et al. MDS and secondary AML display unique patterns and abundance of aberrant DNA methylation. Blood. 2009;114(16):3448–58.
Ball B, Zeidan A, Gore SD, Prebet T. Hypomethylating agent combination strategies in myelodysplastic syndromes: hopes and shortcomings. Leuk Lymphoma. 2017;58(5):1022–36.
Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18(6):553–67.
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–73.
Mardis ER, Ding L. Fau - Dooling DJ, Dooling Dj Fau - Larson DE, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361(11):1058–66.
Marcucci G, Maharry K. Fau - Wu Y-Z, Wu Yz Fau - Radmacher MD, et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a cancer and leukemia group B study. J Clin Oncol. 2010;28(14):2348–55.
Im AP, Sehgal AR, Carroll MP, Smith BD, Tefferi A, Johnson DE, et al. DNMT3A and IDH mutations in acute myeloid leukemia and other myeloid malignancies: associations with prognosis and potential treatment strategies. Leukemia. 2014;28(9):1774–83.
Molenaar RJ, Thota S, Nagata Y, Patel B, Clemente M, Przychodzen B, et al. Clinical and biological implications of ancestral and non-ancestral IDH1 and IDH2 mutations in myeloid neoplasms. Leukemia. 2015;29(11):2134–42.
Popovici-Muller J, Lemieux RM, Artin E, Saunders JO, Salituro FG, Travins J, et al. Discovery of AG-120 (ivosidenib): a first-in-class mutant IDH1 inhibitor for the treatment of IDH1 mutant cancers. ACS Med Chem Lett. 2018;9(4):300–5.
Ward PS, Patel J. Fau - Wise DR, Wise Dr Fau - Abdel-Wahab O, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17(3):225–34. Paper describing mechanism of IDH mutations in AML.
Dang L, Su SM. Isocitrate dehydrogenase mutation and (R)-2-hydroxyglutarate: from basic discovery to therapeutics development. Annu Rev Biochem. 2017;86(1545-4509 (Electronic)):305–31.
Dang L. White Dw Fau - Gross S, Gross S Fau - Bennett BD, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–44. Mechanism of IDH mutations in glioblastoma and description of 2-hydroxyglutarate production.
Yen K, Travins J, Wang F, David MD, Artin E, Straley K, et al. AG-221, a first-in-class therapy targeting acute myeloid leukemia harboring oncogenic IDH2 mutations. Cancer Discov. 2017;7(5):478–93. Paper describing IDH2 inhibitor AG-221 (enasidenib).
Paschka P. Schlenk Rf Fau - Gaidzik VI, Gaidzik Vi Fau - Habdank M, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol. 2010;289(22):3636–43.
Abbas S, Lugthart S. Fau - Kavelaars FG, Kavelaars Fg Fau - Schelen A, et al. Acquired mutations in the genes encoding IDH1 and IDH2 both are recurrent aberrations in acute myeloid leukemia: prevalence and prognostic value. Blood. 2010;116(12):2122–6.
Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079–89.
Dunlap JA-O, Leonard J, Rosenberg M, et al. The combination of NPM1, DNMT3A, and IDH1/2 mutations leads to inferior overall survival in AML. Am J Hematol. 2019;94(8):913–20.
Golub D, Iyengar N, Dogra S, Wong T, Bready D, Tang K, et al. Mutant isocitrate dehydrogenase inhibitors as targeted cancer therapeutics. Front Oncol. 2019;9.
Shih AH, Meydan C, Shank K, Garrett-Bakelman FE, Ward PS, Intlekofer AM, et al. Combination targeted therapy to disrupt aberrant oncogenic signaling and reverse epigenetic dysfunction in IDH2- and TET2-mutant acute myeloid leukemia. Cancer Discov. 2017;7(5):494–505. Combination of hypomethylating agent and IDH inhibitor work together to drive differentiation.
Stein EM, DiNardo CD, Fathi AT, et al. Molecular remission and response patterns in patients with mutant-IDH2 acute myeloid leukemia treated with enasidenib. Blood. 2019;133(7):676–87. Paper describing how IDH2 mutations respond to treatment with enasidenib.
Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722–31. First clinical report of IDH2 inhibitor enasidenib.
Pollyea DA, Tallman MS, de Botton S, Kantarjian HM, Collins R, Stein AS, et al. Enasidenib, an inhibitor of mutant IDH2 proteins, induces durable remissions in older patients with newly diagnosed acute myeloid leukemia. Leukemia. 2019;33(11):2575–84.
DiNardo CD, Stein EM, de Botton S, et al. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N Engl J Med. 2018;378(25):2386–98. First clinical report of IDH1 inhibitor ivosidenib.
Agios announces FDA approval of Supplemental New Drug Application (sNDA) for TIBSOVO as monotherapy for newly diagnosed adult patients with IDH1 mutant acute myeloid leukemia (AML) not eligible for intensive chemotherapy [press release]. Cambridge, MA, May 2 2019.
Roboz GJ, DiNardo CD, Stein EM, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2020;135(7):463–71.
Fau GM, Noetzli J, Fau NJ, Blum S, Fau BS, Duchosal MA, et al. Transfusion independence and survival in patients with acute myeloid leukemia treated with 5-azacytidine. Haematologica. 97(12):1929–31.
Bloomfield CD, Estey E, Pleyer L, Schuh AC, Stein EM, Tallman MS, et al. Time to repeal and replace response criteria for acute myeloid leukemia? Blood Rev. 2018;32(5):416–25.
Fathi AT, DiNardo CD, Kline I, et al. Differentiation syndrome associated with enasidenib, a selective inhibitor of mutant isocitrate dehydrogenase 2: analysis of a phase 1/2 study. JAMA Oncol. 2018;4(8):1106–10.
Montesinos P, Bergua JM, Vellenga E, Rayón C, Parody R, de la Serna J, et al. Differentiation syndrome in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline chemotherapy: characteristics, outcome, and prognostic factors. Blood. 2009;113(4):775–83.
Norsworthy KJ, Luo L, Hsu V, Gudi R, Dorff SE, Przepiorka D, et al. FDA Approval summary: ivosidenib for relapsed or refractory acute myeloid leukemia with an isocitrate dehydrogenase-1 mutation. Clin Cancer Res. 2019;25(11):3205–9.
Norsworthy KJ, Mulkey F, Ward A, et al. Incidence of differentiation syndrome with ivosidenib (IVO) and enasidenib (ENA) for treatment of patients with relapsed or refractory (R/R) isocitrate dehydrogenase (IDH)1- or IDH2-mutated acute myeloid leukemia (AML): a systematic analysis by the U.S. Food and Drug Administration (FDA). Blood. 2018:132–288.
Choe S, Wang H, DiNardo CD, et al. Molecular mechanisms mediating relapse following ivosidenib monotherapy in IDH1-mutant relapsed or refractory AML. Blood Adv. 2020;4(9):1894–905. Clinical description of mechanisms of resistance to IDH inhibitors in large number of patients on trials and frequency of types of resistance.
Amatangelo MD, Quek L, Shih A, Stein EM, Roshal M, David MD, et al. Enasidenib induces acute myeloid leukemia cell differentiation to promote clinical response. Blood. 2017;130(6):732–41.
Quek L, David MD, Kennedy A, Metzner M, Amatangelo M, Shih A, et al. Clonal heterogeneity of acute myeloid leukemia treated with the IDH2 inhibitor enasidenib. Nat Med. 2018;24(8):1167–77.
Harding JJ, Lowery MA, Shih AH, Schvartzman JM, Hou S, Famulare C, et al. Isoform switching as a mechanism of acquired resistance to mutant isocitrate dehydrogenase Inhibition. Cancer Discovery. 2018;8(12):1540–7. Paper describing isoform-switching as mechanism of resistance to IDH inhibitors.
Intlekofer AM, Shih AH, Wang B, Nazir A, Rustenburg AS, Albanese SK, et al. Acquired resistance to IDH inhibition through trans or cis dimer-interface mutations. Nature. 2018;559(7712):125–9. Paper describing resistance mutations as mechanism of resistance to IDH inhibitors.
DiNardo CD, Schuh AC, Stein EM, et al. Enasidenib plus azacitidine significantly improves complete remission and overall response compared with azacitidine alone in patients with newly diagnosed acute myeloid leukemia (AML) with Isocitrate dehydrogenase 2 (IDH2) mutations: interim phase ii results from an ongoing, randomized study. Blood. 2019;134(Supplement_1):643.
A Safety and Efficacy Study of oral AG-120 plus subcutaneous azacitidine and oral AG-221 plus subcutaneous azacitidine in subjects with newly diagnosed acute myeloid leukemia (AML). ClinicalTrials.gov Identifier: NCT02677922. 2019.
DiNardo C, Schuh A, Stein E, et al. Enasidenib plus azacitidine significantly improves complete remission and overall response rates versus azacitidine monotherapy in mutant-IDH2 newly diagnosed acute myeloid leukemia (ND-AML). EHA Library. 2020;294959:S139.
Stein EM, Huang Y, Borate U, et al. 636 Enasidenib (ENA) Monotherapy with addition of azacitidine in non-responders is effective in older patients with newly diagnosed IDH2 mutated acute myeloid leukemia (AML): a completed phase 2/1b sub-study of the beat AML master trial. 62nd ASH Annual Meeting and Exposition 2020;Oral and Poster Abstracts.
DiNardo CD, Stein AS, Stein EM, et al. Mutant isocitrate dehydrogenase 1 inhibitor ivosidenib in combination with azacitidine for newly diagnosed acute myeloid leukemia. J Clin Oncol. 2021;39(1):57–65. Clinical outcomes of ivosidenib and azacytidine combination showing improved response rates compared to single agent.
Fernandez PM, Recher C, Doronin V, et al. AGILE: A phase 3, multicenter, double-blind, randomized, placebo-controlled study of ivosidenib in combination with azacitidine in adult patients with previously untreated acute myeloid leukemia with an IDH1 mutation. Blood. 2019;134(Supplement_1):2593.
Stein E, Dinardo CD, Jang JH, et al. AGILE: a phase 3, multicenter, randomized, placebo-controlled study of ivosidenib in combination with azacitidine in adult patients with previously untreated acute myeloid leukemia with an IDH1 mutation. J Clin Oncol. 2018;36(15_suppl):TPS7074.
Study of AG-120 (Ivosidenib) vs. placebo in combination with azacitidine in patients with previously untreated acute myeloid leukemia with an IDH1 mutation (AGILE). NCT03173248. ClinicalTrials.gov; 2020.
Stein EM, DiNardo CD, Fathi AT, et al. Ivosidenib or enasidenib combined with intensive chemotherapy in patients with newly diagnosed AML: a phase 1 study. Blood. 2020.
Study of biomarker-based treatment of acute myeloid leukemia NCT03013998. ClinicalTrials.gov 2020.
DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–29. Azacitidine and venetoclax combination has activity in multiple subtypes of AML, and high CR rates in IDH mutated AML.
Pan D, Rampal R, Mascarenhas J. Clinical developments in epigenetic-directed therapies in acute myeloid leukemia. Blood Adv. 2020;4(5):970–82.
Stein EM, DiNardo CD, Fathi AT, et al. Ivosidenib or Enasidenib combined with induction and consolidation chemotherapy in patients with newly diagnosed AML with an IDH1 or IDH2 Mutation Is Safe, Effective, and Leads to MRD-Negative Complete remissions. Blood. 2018;132(Supplement 1):560.
DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17.
Curtis Andrew Lachowiez GB, Loghavi S, Zeng Z, Kadia TM, Masarova L, Takahashi K, et al. Phase Ib/II study of the IDH1-mutant inhibitor ivosidenib with the BCL2 inhibitor venetoclax +/- azacitidine in IDH1-mutated hematologic malignancies. J Clin Oncol. 2020;38(suppl; abstr 7500):2020.
Acknowledgements
The authors would like to acknowledge our patients treated with IDH inhibitors. We also thank Willow Traer for her rendering of the mitochondria and nucleus in Fig. 1.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this work.
Corresponding author
Ethics declarations
Conflict of Interest
E. Traer potential competing interests: advisory board/consulting: Abbvie, Agios, Astellas, Daiichi-Sankyo. Clinical trial funding: Janssen, Incyte, LLS BeatAML. Stock options: notable Labs. H. McMurry and L. Fletcher declare that they have no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Acute Myeloid Leukemias
Rights and permissions
About this article
Cite this article
McMurry, H., Fletcher, L. & Traer, E. IDH Inhibitors in AML—Promise and Pitfalls. Curr Hematol Malig Rep 16, 207–217 (2021). https://doi.org/10.1007/s11899-021-00619-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-021-00619-3