Abstract
Purpose of Review
Obesity is a major driver of heart failure (HF) incidence, and aggravates its pathophysiology. We summarized key reported and ongoing randomized clinical trials of appetite regulation and/or dietary energy restriction in individuals with HF.
Recent Findings
Weight loss can be achieved by structured supervised diet programs with behavioural change, medications, or surgery. The new glucagon-like peptide-1 receptor agonists alone or in combination with other agents (e.g., glucose-dependent insulinotropic polypeptide and glucagon receptor agonists or amylin analogues) potently and sustainably reduce appetite, and, taken together with dietary advice, can produce substantial, life-changing, weight loss approaching that achieved by surgery. To date, data from the STEP-HFpEF trial show meaningful improvements in health status (Kansas City Cardiomyopathy Questionnaire).
Summary
Effective weight management could relieve several drivers of HF, to complement the existing treatments for HF with both reduced and preserved ejection fraction. Further trials of weight loss interventions will provide more definitive evidence to understand their effects on clinical events in patients with HF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Obesity and Heart Failure (HF)
Obesity increases metabolic rate and demand on the cardiovascular and respiratory systems, is a major driver of HF incidence, and aggravates its pathophysiology: the heart, like any machine, will fail if the systemic demand or load is too great. Combined, HF and obesity cause an especially poor quality of life, with more HF hospitalizations and cardiovascular deaths [1, 2].
Appetite Regulation
Appetite, defined as a desire for food, is regulated centrally by the hypothalamus, and by several hormones secreted peripherally, some in response to dietary intake.
Weight Loss
While unintentional weight loss usually indicates disease and poor prognosis, modifying appetite to induce negative caloric (energy) balance generates intentional weight loss, appears to be an efficacious treatment option for people with HF and obesity, to treat or alleviate HF and comorbidities [3,4,5]. Weight loss is possible with structured supervised diet programs with behavioural change, but only sustainable if appetite can be overcome or altered, by professional and lay support, changes in food choices, medications, or surgery [6]. Whilst bariatric surgery usually generates major weight loss, it is a more challenging option in the presence of HF, and late weight regain and side-effects (e.g. dumping syndrome, vitamin and mineral deficiencies, need for re-operation or reversal) are common problems. Evidence-based diet programmes including nutritional formula ‘total diet replacement’ for the weight loss phase can reliably produce weight loss > 10 kg at 12 months [6]. The new GLP-1 receptor agonists, GIP and glucagon receptor agonists, potently and sustainably reduce appetite, and, taken together with dietary advice, can produce substantial, life-changing, weight loss approaching that achieved by surgery [7, 8]. Are these strategies ‘palatable’ to individuals with HF and to healthcare systems? We recently suggested targeting more conditions earlier with weight loss may reap multiple gains (including reducing multimorbidity) beyond currently recommended treatment options [3].
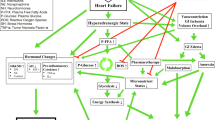
Reported and Ongoing Clinical Trials (Fig. 1)
In HF with preserved ejection fraction (HFpEF), the largest diet-driven randomised trial (SECRET; n = 100) of a dietary intervention (caloric restriction (provision of all foods in a university setting)) resulted in 7 kg weight loss and improved 6-min walk distance, peak oxygen uptake, health status, and regression in left ventricular mass, compared to those who did not undergo the diet intervention [4]. Based on this one trial, caloric restriction is listed as a treatment option in international guidelines and expert consensus documents [9, 10]. Other diet trials in HFpEF were smaller, non-randomised, or achieved only modest weight loss (< 10 kg) [11, 12]. In STEP-HFpEF (n = 529) the GLP-1 receptor agonist semaglutide reduced weight by 10.7 %, improved health status, reduced a hierarchical composite endpoint, improved exercise function, and reduced inflammation and NT-proBNP [5]. In HF with reduced ejection fraction (HFrEF), dietary studies have been small or non-randomised [13]. In HFrEF, two trials in non-obese populations of the GLP-1 receptor agonist liraglutide achieved trivial weight loss (~ 2 kg); there were very small numbers of clinical endpoints in these trials, rendering unclear conclusions [14, 15]. There are no randomized trials of bariatric surgery in HFpEF or HFrEF, only observational evidence that supports a potential benefit from intentional weight loss [16]. The ongoing BRAVE open-label randomized controlled trial (NCT04226664) is assessing bariatric surgery versus medical weight management and will recruit ~ 2000 patients with high-risk cardiovascular disease (including, importantly, a subgroup (interim data n = 17/49 (35%)) with symptomatic HF) [17] (Fig. 1).
Key randomized trials of appetite regulation in HF (key outcomes; green = completed; yellow = ongoing; between-group differences shown except for SECRET-II where within-group differences shown). Abbreviations: 6MWD, 6-min walk distance; AT, aerobic exercise training; CR, caloric restriction; CRP, C-reactive protein; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1; HF, heart failure; HFH, heart failure hospitalization; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; KCCQ-CSS, Kansas City Cardiomyopathy Questionnaire Clinical Summary Score; LAVI, left atrial volume index; LVEF, left ventricular ejection fraction; LVM, left ventricular mass; MRI, magnetic resonance imaging; NS, not significant; NT-proBNP, N-terminal pro-B-type natriuretic peptide; pVO2, peak oxygen uptake; RT, resistance training; wt, body weight
Future Directions
From first principles, earlier, effective weight management with substantial weight loss should relieve several drivers of HF, to complement the existing treatments for HFrEF and potentially provide a vital effective intervention for HFpEF. Adequately powered outcome trials are needed to provide definitive evidence, to understand the effect of intentional weight loss on clinical events, and mechanisms of benefit. The value of combining therapies must be assessed: GLP-1/GIP/glucagon receptor agonists appear promising adjunctive treatments that warrant definitive trials, and it remains possible that some effects of these medications may be independent of weight loss. Challenges include long-term weight-loss maintenance, programme accessibility, flexibility and adherence, and costs. Hippocrates reminded us: “If we could give every individual the right amount of nourishment and exercise, not too little and not too much, we would have found the safest way to health.” [18] Healthcare systems need to target weight loss much more actively for societal and patient gains [3].
Data Availability
All supporting data are available within the cited references. No new data were generated in support of this review.
References
Reddy YNV, Rikhi A, Obokata M, et al. Quality of life in heart failure with preserved ejection fraction: importance of obesity, functional capacity, and physical inactivity. Eur J Heart Fail. 2020;22(6):1009–18. https://doi.org/10.1002/ejhf.1788.
Butt JH, Petrie MC, Jhund PS, et al. Anthropometric measures and adverse outcomes in heart failure with reduced ejection fraction: revisiting the obesity paradox. Eur Heart J. 2023;44(13):1136–53. https://doi.org/10.1093/eurheartj/ehad083.
Sattar N, McMurray JJV, McInnes IB, Aroda VR, Lean MEJ. Treating chronic diseases without tackling excess adiposity promotes multimorbidity. Lancet Diabetes Endocrinol. 2023;11(1):58–62. https://doi.org/10.1016/S2213-8587(22)00317-5.
Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2016;315(1):36–46. https://doi.org/10.1001/jama.2015.17346.
Kosiborod MN, Abildstrøm SZ, Borlaug BA, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med. 2023;389(12):1069–84. https://doi.org/10.1056/NEJMoa2306963.
Lean MEJ, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391(10120):541–51. https://doi.org/10.1016/S0140-6736(17)33102-1.
Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. https://doi.org/10.1056/NEJMoa2032183.
Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–16. https://doi.org/10.1056/NEJMoa2206038.
McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726. https://doi.org/10.1093/eurheartj/ehab368.
Kittleson MM, Panjrath GS, Amancherla K, et al. 2023 ACC expert consensus decision pathway on management of heart failure with preserved ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2023;81(18):1835–78. https://doi.org/10.1016/j.jacc.2023.03.393.
Forsyth F, Mulrennan S, Burt J, et al. What dietary interventions have been tested in heart failure with preserved ejection fraction? A systematic scoping review. Eur J Cardiovasc Nurs. 2023;22(2):126–40. https://doi.org/10.1093/eurjcn/zvac062.
Brubaker PH, Nicklas BJ, Houston DK, et al. A randomized, controlled trial of resistance training added to caloric restriction plus aerobic exercise training in obese heart failure with preserved ejection fraction. Circ Heart Fail. 2023;16(2):e010161. https://doi.org/10.1161/CIRCHEARTFAILURE.122.010161.
McDowell K, Petrie MC, Raihan NA, Logue J. Effects of intentional weight loss in patients with obesity and heart failure: a systematic review. Obes Rev. 2018;19(9):1189–204. https://doi.org/10.1111/obr.12707.
Jorsal A, Kistorp C, Holmager P, et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur J Heart Fail. 2017;19(1):69–77. https://doi.org/10.1002/ejhf.657.
Margulies KB, Hernandez AF, Redfield MM, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2016;316(5):500–8. https://doi.org/10.1001/jama.2016.10260.
Höskuldsdóttir G, Sattar N, Miftaraj M, et al. Potential effects of bariatric surgery on the incidence of heart failure and atrial fibrillation in patients with type 2 diabetes mellitus and obesity and on mortality in patients with preexisting heart failure: a nationwide, matched, observational cohort study. J Am Heart Assoc. 2021;10(7):e019323. https://doi.org/10.1161/JAHA.120.019323.
PHRI. BRAVE. Published 2023. https://www.phri.ca/research/brave/. Accessed 31 Oct 2023.
Hippocrates. Hippocratic writings. Encyclopedia Britannica. 1955. https://books.google.co.uk/books/about/Hippocratic_Writings.html?id=OxQxzgEACAAJ&redir_esc=y.
Funding
Sattar and Petrie are supported by a British Heart Foundation Centre of Research Excellence Grant (RE/18/6/34217).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Lee received research grants through his institution, the University of Glasgow, from AstraZeneca, Boehringer Ingelheim, and Roche Diagnostics; is a member of a Trial Steering Committee for Cytokinetics, and Clinical Endpoints Committee for Bayer.
Lean declares personal fees for lecturing from Novo Nordisk, Nestle, and Eli Lilly; and departmental funding support for research and consultancy from Novo Nordisk, Counterweight Ltd, Diabetes UK, All Saints Educational Trust, and NIHR.
Sattar received consulting/speaker honoraria from Abbott Laboratories, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceuticals, Janssen, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, and Sanofi; received grants from AstraZeneca, Boehringer Ingelheim, Novartis, and Roche Diagnostics.
Petrie received research funding from Boehringer Ingelheim, Roche, SQ Innovations, AstraZeneca, Novartis, Novo Nordisk, Medtronic, Boston Scientific, Pharmacosmos; participation in Consultancy and Trial committees for Akero, Applied Therapeutics, AnaCardio, Biosensors, Boehringer Ingelheim, Novartis, AstraZeneca, Novo Nordisk, Abbvie, Bayer, Horizon Therapeutics, Takeda, Cardiorentis, Pharmacosmos, Siemens, Eli Lilly, Vifor, New Amsterdam, Moderna, and Teikoku.
Human and Animal Rights and Informed Consent
All reported studies with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards and international/national/institutional guidelines).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, M.M.Y., Lean, M.E.J., Sattar, N. et al. Appetite and its Regulation: Are there Palatable Interventions for Heart Failure?. Curr Heart Fail Rep 21, 1–4 (2024). https://doi.org/10.1007/s11897-023-00637-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-023-00637-7