Abstract
Purpose of Review
The purpose of this review was to review advances in basal insulin formulations and new treatment options for patients with type 2 diabetes not achieving glycemic targets despite optimized basal insulin therapy.
Recent Findings
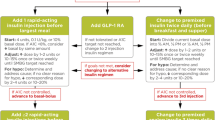
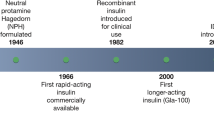
Advances in basal insulin formulations have resulted in products with increasingly favorable pharmacokinetic and pharmacodynamic properties, including flatter, peakless action profiles, less inter- and intra-patient variability, and longer duration of activity. These properties have translated to significantly reduced risk of hypoglycemia (particularly during the night) compared with previous generation basal insulins. When optimized basal insulin therapy is not sufficient to obtain or maintain glycemic goals, various options exist to improve glycemic control, including intensification of insulin therapy with the addition of prandial insulin or changing to pre-mixed insulin and, more recently, the addition of a GLP-1 receptor agonist, either as a separate injection or as a component of one of the new fixed-ratio combinations of a basal insulin and GLP-1 RA.
Summary
New safer and often more convenient basal insulins and fixed ratio combinations containing basal insulin (and GLP-1 receptor agonist) are available today for patients with type 2 diabetes not achieving glycemic goals. Head-to-head studies comparing the latest generation basal insulins are underway, and future studies assessing the fixed-ratio combinations will be important to better understand their differentiating features.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Shulman G, Barrett E, Sherwin R. Integrated fuel metabolism. In: Porte D, Sherwin R, Baron A, editors. Ellenberg and Rifkin’s Diabetes Mellitus: The McGraw-Hill Companies; 2003.
Ahern B, Taborsky GJ. Beta-cell function and insulin secretion. In: Porte D, Sherwin R, Baron A, editors. Ellenberg and Rifkin’s Diabetes Mellitus: The McGraw-Hill Companies; 2003.
DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–95.
Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Beta cell function in subjects spanning the range from normal glucose tolerance to overt diabetes mellitus: a new analysis. J Clin Endocrinol Metab. 2005;90:493–500.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American diabetes association and the European association for the study of diabetes. Diabetes Care. 2015;38:140–9.
American Diabetes Association. Pharmacologic approaches to glycemic treatment. Sec 8. In standards of medical Care in Diabetes – 2017. Diabetes Care. 2017;40(Suppl. 1):S64–74.
Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. AACE/ACE comprehensive diabetes management algorithm 2015. Endocr Pract. 2016;22:84–113.
• Rosenstock J, Aronson R, Grunberger G, Hanefeld M, Piotti P, Sersclat P, et al. Benefits of LixiLan, a titratable fixed-ratio combination of insulin glargine plus Lixisenatide, versus insulin glargine and Lixisenatide Monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan-O randomized trial. Diabetes Care. 2016;39:2026–35. This study demonstrates the benefits of the fixed-ratio combination IGlarLixi versus IGlar U-100 and lixisenatide in patients with type 2 diabetes suboptimally controlled on oral antidiabetic agents.
• Aroda V, Rosenstock J, Wysham C, Unger J, Bellido D, Gonzalez-Galvez G, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of insulin glargine plus Lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L randomized trial. Diabetes Care. 2015;39:1972–80. This study demonstrates the benefits of the fixed-ratio combination IGlarLixi versus IGlar U-100 in patients with type 2 diabetes suboptimally controlled on basal insulin with or without oral antidiabetic agents.
• Gough SC, Bode B, Woo V, Rodbard HW, Linjawi S, Poulsen P, et al. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2:885–93. This study demonstrates the benefits of the fixed-ratio combination IDegLira versus IDeg and liraglutide in patients with type 2 diabetes suboptimally controlled on oral antidiabetic agents.
• Buse J, Vilsbøll T, Thurman J, Blevins T, Langbakke I, Bøttcher S, et al. Contribution of Liraglutide in the fixed-ratio combination of insulin Degludec and Liraglutide (IDegLira). Diabetes Care. 2014;37:2926–33. This study demonstrates the benefits of the fixed-ratio combination IDegLira versus IDeg in patients with type 2 diabetes suboptimally controlled on basal insulin with or without oral antidiabetic agents.
Lantus [package insert]. Bridgewater. Sanofi-Aventis; 2015.
Levemir [package insert]. Princeton: Novo Nordisk; 2005.
Toujeo [package insert]. Bridgewater: Sanofi-Aventis; 2015.
Tresiba [package insert]. Plainsboro: Novo Nordisk; 2016.
Rosenstock J, Dailey G, Massi-Benedetti M, Fritsche A, Lin Z, Salzman A. Reduced hypoglycemia risk with insulin glargine: a meta-analysis comparing insulin glargine with human NPH insulin in type 2 diabetes. Diabetes Care. 2005;28:950–5.
Garber AJ, Clauson P, Pedersen CB, Kølendorf K. Lower risk of hypoglycemia with insulin Detemir than with neutral protamine Hagedorn insulin in older persons with type 2 diabetes: a pooled analysis of phase III trials. J Am Geriatr Soc. 2007;55:1735–40.
Ritzel R, Roussel R, Bolli GB, Vinet L, Brulle-Wohlhueter C, Glezer S, et al. Patient-level meta-analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab. 2015;17:859–67.
Ratner RE, Gough SC, Mathieu C, Del Prato S, Bode B, Mersebach H, et al. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre-planned meta-analysis of phase 3 trials. Diabetes Obes Metab. 2013;15:175–84.
Sorli C, Warren M, Oyer D, Mersebach H, Johansen T, Gough SC. Elderly patients with diabetes experience a lower rate of nocturnal hypoglycaemia with insulin degludec than with insulin glargine: a meta-analysis of phase IIIa trials. Drugs Aging. 2013;30:1009–18.
Søeborg T, Rasmussen CH, Mosekilde E, Colding-Jørgensen M. Absorption kinetics of insulin after subcutaneous administration. Eur J Pharm Sci. 2009;36:78–90.
Heise T, Mathieu C. Impact of the mode of protraction of basal insulin therapies on their pharmacokinetic and pharmacodynamic properties and resulting clinical outcomes. Diabetes Obes Metab. 2017;19:3–12.
Lucidi P, Porcellati F, Andreoli AM, Carriero I, Candeloro P, Cioli P, et al. Pharmacokinetics and pharmacodynamics of NPH insulin in type 1 diabetes: the importance of appropriate resuspension before subcutaneous injection. Diabetes Care. 2015;38:2204–10.
Humulin® N [package insert]. Indianapolis : Eli Lilly & CO; 2015.
Novolin® N [package insert]. Princeton: Novo Nordisk A/S; 2010.
Hilgenfeld R, Seipke G, Berchtold H, Owens DR. The evolution of insulin glargine and its continuing contribution to diabetes care. Drugs. 2014;74:911–27.
Owens DR. Insulin preparations with prolonged effect. Diab Tech and Thera. 2011;13(Suppl 1):S5–S14.
Basiglar [package insert]. Indianapolis. Eli Lilly & CO; 2016.
Rosenstock J, Hollander P, Bhargava A, Ilag LL, Pollom RK, Zielonka JS, et al. Similar efficacy and safety of LY2963016 insulin glargine and insulin glargine (Lantus®) in patients with type 2 diabetes who were insulin-naïve or previously treated with insulin glargine: a randomized, double-blind controlled trial (the ELEMENT 2 study). Diabetes Obes Metab. 2015;17:734–41.
Clincaltrial.gov NCT02059187 and NCT02227875 Accessed May 19, 2017.
Havelund S, Plum A, Ribel U, Jonassen I, Vølund A, Markussen J, et al. The mechanism of protraction of insulin detemir, a long-acting, acylated analog of human insulin. Pharm Res. 2004;21:1498–504.
Becker RH, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 units.mL−1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 units.mL−1. Diabetes Care. 2015;38:637–43.
Drab SR, Philis-Tsimikas A. A new option for glycemic control: insulin degludec, a new-generation basal insulin with an ultralong duration of action. Pharmacotherapy. 2014;34:291–302.
Heise T, Norskov M, Nosek L, Kaplan K, Famulla S, Haahr HL. Insulin degludec: lower day-to-day and within-day variability in pharmacodynamic response compared with insulin glargine 300 U/mL in type 1 diabetes. Diabetes Obes Metab. 2017;19(7):1032–9.
Riddle M, Rosenstock J, Gerich J. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080–6.
Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care. 2006;29:1269–74.
Rosenstock J, Davies M, Home P, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia. 2008;51:408–16.
• Riddle MC, Bolli GB, Ziemen M, Muehlen-Bartmer I, Bizet F, Home PD. EDITION 1 study investigators. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 1). Diabetes Care. 2014;37:2755–62. This study demonstrates the benefits of IGlar U-300 versus IGlar U-100 in patient with type 2 diabetes.
Yki-Jarvinen H, Bergenstal R, Ziemen M, Wardecki M, Muehlen-Bartmer I, Boelle E, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care. 2014;37:3235–43.
Bolli GB, Riddle MC, Bergenstal RM, Ziemen M, Sestakauskas K, Goyeau H, et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naive people with type 2 diabetes on oral glucose lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab. 2015;17:386–94.
• Zinman B, Philis-Tsimikas A, Cariou B, Handelsman Y, Rodbard HW, Johansen T, et al. Insulin degludec versus insulin glargine in insulin-naive patients with type 2 diabetes: a 1-year, randomized, treat-to-target trial (BEGIN once long). Diabetes Care. 2012;35:2464–71. This study demonstrates the benefits of IDeg versus IGlar U-100 in patient with type 2 diabetes.
Garber AJ, King AB, Del Prato S, Sreenan S, Balci MK, Muñoz-Torres M, et al. Insulin degludec, an ultra-long-acting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN basal-bolus type 2): a phase 3, randomized, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379:1498–507.
Wysham C, Bhargava A, Chaykin L, de la Rosa R, Handelsman Y, Troelsen L, et al. Effects of insulin degludec vs insulin glargine U100 of hypoglycemia in patients with type 2 diabetes. The SWITCH 2 randomized clinical trial. JAMA. 2017;318:45–56.
Meneghini L, Atkin SL, Gough SC, Raz I, Blonde L, Shestakova M, et al. The efficacy and safety of insulin degludec given in variable once-daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily: a 26-week, randomized, open-label, parallel-group, treat-to-target trial in individuals with type 2 diabetes. Diabetes Care. 2013;36:858–64.
•• Marso SP, McGuire DK, Zinman B, Poulter NR, Emerson SS, Pieber TR, et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med. 2017; doi:10.1056/NEJMoa1615692. This long-term, large-scale study demonstrates the cardiovascular safety of IDeg, as well as lower risk of severe hypoglycemia versus IGlar U-100 in patients with type 2 diabetes.
Clincaltrial.gov NCT02738151 and NCT03078478 Accessed May 19, 2017.
Lambert K, Hold RIG. The use of insulin analogues in pregnancy. Diabetes Obes Metab. 2013;15:888–900.
Toledano Y, Hadar E, Hod M. Safety of insulin analogues as compared with human insulin in pregnancy. Expert Opin Drug Saf. 2016;15:963–73.
Shank ML, Del Prato S, DeFronzo RA. Bedtime insulin/daytime glipizide. Effective therapy for sulfonylurea failures in NIDDM. Diabetes. 1995;44:165–72.
Holman R, Thorne K, Farmer A, Davies M, Keenan J, Paul S, et al. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med. 2007;357:1716–30.
Holman R, Farmer A, Davies M, Levy J, Darbyshire J, Keenan J, et al. For the 4-T study group three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361:1736–47.
Anderson SL, Trujillo JM. Basal insulin use with GLP-1 receptor agonists. Diabetes Spectrum. 2016;29:152–60.
Esposito K, Chiodini P, Bellastella G, Maiorino MI, Giugliano D. Proportion of patients at HbA1c target <7% with eight classes of antidiabetic drugs in type 2 diabetes: systematic review of 218 randomized controlled trials with 78,945 patients. Diabetes Obes Metab. 2012;14:228–33.
Dalal MR, Grabner M, Bonine N, Stephenson JJ, DiGenio A, Bieszk N. Are patients on basal insulin attaining glycemic targets? Characteristics and goal achievement of patients with type 2 diabetes mellitus treated with basal insulin and physician-perceived barriers to achieving glycemic targets. Diabetes Res Clin Pract. 2016;121:17–26.
Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384:2228–34.
Berlie H, Hurren KM, Pinelli NR. Glucagon-like peptide-1 receptor agonists as add-on therapy to basal insulin in patients with type 2 diabetes: a systematic review. Diabetes Metab Syndr Obes. 2012;5:165–74.
Soliqua [package insert]. Bridgewater: Sanofi-Aventis; 2016.
Xultophy [package insert]. Plainsboro: Novo Nordisk; 2016.
Suliqua [package insert]. Paris: Sanofi-Aventis; 2017.
Xultophy [package insert]. Bagsvaerd: Novo Nordisk; 2014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Patrick F. Frias declares no conflict of interest.
Juan Pablo Frias reports grants and personal fees from Sanofi, grants and personal fees from Novo Nordisk, and grants from Eli Lilly, Merck, and Mylan.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable standards (including the Helsinki declaration and its amendments, instructional/national research committee standards, and international/national/institutional guidelines).
Additional information
This article is part of the Topical Collection on Pharmacologic Treatment of Type 2 Diabetes
Rights and permissions
About this article
Cite this article
Frias, P.F., Frias, J.P. New Basal Insulins: a Clinical Perspective of Their Use in the Treatment of Type 2 Diabetes and Novel Treatment Options Beyond Basal Insulin. Curr Diab Rep 17, 91 (2017). https://doi.org/10.1007/s11892-017-0926-8
Published:
DOI: https://doi.org/10.1007/s11892-017-0926-8