Abstract
Purpose of Review
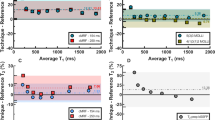
Cardiac magnetic resonance fingerprinting (cMRF) has developed as a technique for rapid, multi-parametric tissue property mapping that has potential to both improve cardiac MRI exam efficiency and expand the information captured. In this review, we describe the cMRF technique, summarize technical developments and in vivo reports, and highlight potential clinical applications.
Recent Findings
Technical developments in cMRF continue to progress rapidly, including motion compensated reconstruction, additional tissue property quantification, signal time course analysis, and synthetic LGE image generation. Such technical developments can enable simplified CMR protocols by combining multiple evaluations into a single protocol and reducing the number of breath-held scans. cMRF continues to be reported for use in a range of pathologies; however barriers to clinical implementation remain.
Summary
Technical developments are described in this review, followed by a focus on potential clinical applications that they may support. Clinical translation of cMRF could shorten protocols, improve CMR accessibility, and provide additional information as compared to conventional cardiac parametric mapping methods. Current needs for clinical implementation are discussed, as well as how those needs may be met in order to bring cMRF from its current research setting to become a viable tool for patient care.







Similar content being viewed by others
Data Availability
The data that support this review follow the availability policies of referenced articles or are available upon reasonable request to the corresponding author, DHK. The data that require a request are not publicly available due to policies to protect the privacy of study participants
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Ma D, et al. Magnetic resonance fingerprinting. Nature. 2013;495(7440):187–92.
Poorman ME, et al. Magnetic resonance fingerprinting part 1: Potential uses, current challenges, and recommendations. J Magn Reson Imaging. 2020;51(3):675–92.
McGivney DF, et al. Magnetic resonance fingerprinting review part 2: Technique and directions. J Magn Reson Imaging. 2020;51(4):993–1007.
Jiang Y, et al. MR fingerprinting using fast imaging with steady state precession (FISP) with spiral readout. Magn Reson Med. 2015;74(6):1621–31.
Eck BL, et al. Cardiac magnetic resonance fingerprinting: Trends in technical development and potential clinical applications. Prog Nucl Magn Reson Spectrosc. 2021;122:11–22.
Hamilton JI, et al. MR fingerprinting for rapid quantification of myocardial T1, T2, and proton spin density. Magn Reson Med. 2017;77(4):1446–58.
Liu Y, et al. Cardiac magnetic resonance fingerprinting: Technical overview and initial results. JACC: Cardiovasc Imaging. 2018;11(12):1837–53.
Cruz G, et al. Cardiac magnetic resonance fingerprinting: Technical developments and initial clinical validation. Curr Cardiol Rep. 2019;21(9):1–10.
Hamilton JI, et al. Cardiac MR fingerprinting for T1 and T2 mapping in four heartbeats. J Cardiovasc Magn Reson. 2016;18(1):1–3.
Hamilton JI, et al. Low rank compressed sensing reconstruction for more precise cardiac MRF measurements. In Proceedings of the 25th Scientific Meeting of ISMRM, Honolulu, HI. 2017.
Lima da Cruz G, et al. Sparsity and locally low rank regularization for MR fingerprinting. Magn Reson Med. 2019;81(6):3530–43.
Hamilton JI, et al. Investigating and reducing the effects of confounding factors for robust T1 and T2 mapping with cardiac MR fingerprinting. Magn Reson Imaging. 2018;53:40–51.
• Lima da Cruz GJ, et al. Myocardial T1, T2, T2*, and fat fraction quantification via low‐rank motion‐corrected cardiac MR fingerprinting. Magn Reson Med. 2022;87(6):2757–74. This study demonstrates the use of cMRF to provide simultaneous T1, T2, T2*, and fat fraction maps in a single breath-held scan that may be useful for both simplifying cardiac MRI protocols as well as capturing greater information. Furthermore, it describes a framework for motion compensated reconstruction that is likely to be needed when deploying cMRF in real-world settings where some patients may struggle with breath-holds.
Hamilton JI, et al. Simultaneous multislice cardiac magnetic resonance fingerprinting using low rank reconstruction. NMR Biomed. 2019;32(2):e4041.
Hamilton JI, et al. Cardiac cine magnetic resonance fingerprinting for combined ejection fraction, T1 and T2 quantification. NMR Biomed. 2020;33(8):e4323.
Jaubert O, et al. Free-running cardiac magnetic resonance fingerprinting: Joint T1/T2 map and Cine imaging. Magn Reson Imaging. 2020;68:173–82.
• Cruz G, et al. 3D free-breathing cardiac magnetic resonance fingerprinting. NMR Biomed. 2020;33(10):e4370. This report describes and demonstrates a 3D, free-breathing acquisition and reconstruction method that includes respiratory motion compensation for simultaneous T1 and T2 mapping by cMRF. Such an acquisition and reconstruction scheme could substantially simplify cardiac MRI protocols and avoid challenges involved in breath-held scanning methods.
Rashid I, et al. Synthetic multi-contrast late gadolinium enhancement using post-contrast cardiac MR fingerprinting. in Joint Annual Meeting ISMRM-ESMRMB & ISMRT. London, England. 2022.
Jaubert O, et al. Water–fat Dixon cardiac magnetic resonance fingerprinting. Magn Reson Med. 2020;83(6):2107–23.
Liu Y, et al. Myocardial T1 and T2 quantification and water–fat separation using cardiac MR fingerprinting with rosette trajectories at 3T and 1.5 T. Magn Reson Med. 2021;85(1):103–19.
Velasco C, et al. Simultaneous T1, T2, and T1ρ cardiac magnetic resonance fingerprinting for contrast agent–free myocardial tissue characterization. Magn Reson Med. 2022;87(4):1992–2002.
Hamilton JI, Seiberlich N. Machine learning for rapid magnetic resonance fingerprinting tissue property quantification. Proc IEEE. 2019;108(1):69–85.
• Fyrdahl A, Seiberlich N, Hamilton JI. Magnetic resonance fingerprinting: The role of artificial intelligence. In Artificial Intelligence in Cardiothoracic Imaging. Springer. 2022;201–15. This review provides a comprehensive description of potential uses of artificial intelligence in cMRF, as well as a thorough background on cMRF-based tissue property mapping itself.
Hamilton JI. A Self-supervised deep learning reconstruction for shortening the breathhold and acquisition window in cardiac magnetic resonance fingerprinting. Front Cardiovasc Med. 2022;1665.
• Eck BL, et al. Characterization of cardiac amyloidosis using cardiac magnetic resonance fingerprinting. Int J Cardiol. 2022;351:107–10. This report describes the use of cMRF for disease classification, both in the conventional T1 and T2 mapping perspective as well as a cMRF signal timecourse perspective. Such use of cMRF signal timecourses is unique to cMRF as compared to conventional mapping methods and this initial report suggests that it may provide additional benefit over T1 and T2 mapping alone.
• Hamilton JI, et al. Simultaneous mapping of T1 and T2 using cardiac magnetic resonance fingerprinting in a cohort of healthy subjects at 1. 5T. J Magn Reson Imaging. 2020;52(4):1044–52. This is the most comprehensive report to date of cMRF in subjects with no known cardiovascular disease, including comparisons to conventional T1 and T2 mapping methods. This report shows that although cMRF had slightly lower precision as conventional mapping, cMRF did have comparable test-retest and intra-reader repeatability as well as superior image quality ratings for various image features.
Liu Y, et al. Inter-site reproducibility of cardiac magnetic resonance fingerprinting T1 and T2 quantification in the ISMRM/NIST MRI system phantom and human heart. Proc Sci Meet Int Soc Magn Reson Med Montr. 2019;4456.
Rajagopalan V, et al. Performance of cardiac magnetic resonance fingerprinting mapping-evaluation by comparison with standard techniques in a large patient population. Circulation. 2021;144(Suppl_1):A14232–A14232.
Eck B, et al. Comparison of myocardial T1 mapping from cardiac magnetic resonance fingerprinting to MOLLI for translation into clinical protocols. Soc Cardiovasc Magn Reson Annu Meet. 2022.
Cohen JA, et al. Association of cardiac MR Fingerprinting derived T1 and T2 values with global left ventricular strain in patients with cardiac amyloidosis. Soc Cardiovasc Magn Reson Annu Meet. 2022.
Cavallo AU, et al. CMR fingerprinting for myocardial T1, T2, and ECV quantification in patients with nonischemic cardiomyopathy. JACC: Cardiovasc Imaging. 2019;12(8 Part 1):1584–5.
Wintersperger BJ, et al. Quantitative multiparametric myocardial evaluation in hypertrophic cardiomyopathy using cardiac magnetic resonance fingerprinting: Comparison to conventional cardiac relaxometry. Proc Sci Meet Int Soc Magn Reson Med Montr. 2019;2022.
Vincenti G, et al. Cardiac magnetic resonance fingerprinting for the investigation of suspected inflammatory cardiomyopathy. Proc Sci Meet Int Soc Magn Reson Med Montr. 2019;781.
Coristine AJ, et al. Cardiac magnetic resonance fingerprinting in heart transplant recipients. Proc Sci Meet Int Soc Magn Reson Med Paris. 2018;675.
Ma D, et al. Music-based magnetic resonance fingerprinting to improve patient comfort during MRI examinations. Magn Reson Med. 2016;75(6):2303–14.
Eck BL, et al. Feasibility of magnetic resonance fingerprinting on aging MRI hardware. Tomography. 2021;8(1):10–21.
Fontana M, et al. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2015;132(16):1570–9.
Ridouani F, et al. Myocardial native T2 measurement to differentiate light-chain and transthyretin cardiac amyloidosis and assess prognosis. J Cardiovasc Magn Reson. 2018;20(1):1–11.
Kotecha T, et al. Myocardial edema and prognosis in amyloidosis. J Am Coll Cardiol. 2018;71(25):2919–31.
Patel RK, Fontana M, Ruberg FL. Cardiac amyloidosis: Multimodal imaging of disease activity and response to treatment. Circ Cardiovasc Imaging. 2021;14(6):e009025.
Ferreira VM, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: Expert recommendations. J Am Coll Cardiol. 2018;72(24):3158–76.
Gräni C, et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol. 2017;70(16):1964–76.
Radunski UK, et al. T1 and T2 mapping cardiovascular magnetic resonance imaging techniques reveal unapparent myocardial injury in patients with myocarditis. Clin Res Cardiol. 2017;106(1):10–7.
Bönner F, et al. Myocardial T2 mapping increases noninvasive diagnostic accuracy for biopsy-proven myocarditis. JACC: Cardiovasc Imaging. 2016;9(12):1467–9.
Greulich S, et al. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC: Cardiovasc Imaging. 2013;4:501–11.
Chetrit M, et al. Imaging-guided therapies for pericardial diseases. Cardiovasc Imaging. 2020;13(6):1422–37.
Baessler B, et al. Cardiac MRI and texture analysis of myocardial T1 and T2 maps in myocarditis with acute versus chronic symptoms of heart failure. Radiology. 2019;292(3):608–17.
Baessler B, et al. Cardiac MRI texture analysis of T1 and T2 maps in patients with infarctlike acute myocarditis. Radiology. 2018;289(2):357–65.
Dastmalchian S, et al. Radiomic analysis of magnetic resonance fingerprinting in adult brain tumors. Eur J Nucl Med Mol Imaging. 2021;48(3):683–93.
Fujita S, et al. Radiomics with 3-dimensional magnetic resonance fingerprinting: Influence of dictionary design on repeatability and reproducibility of radiomic features. Eur Radiol. 2022;1–10.
Schefold JC, et al. Heart failure and kidney dysfunction: Epidemiology, mechanisms and management. Nat Rev Nephrol. 2016;12(10):610–23.
Do C, et al. Gadolinium-based contrast agent use, their safety, and practice evolution. Kidney360. 2020;1(6):561.
Captur G, et al. T1 mapping performance and measurement repeatability: Results from the multi-national T1 mapping standardization phantom program (T1MES). J Cardiovasc Magn Reson. 2020;22(1):1–17.
Weingärtner S, et al. Development, validation, qualification, and dissemination of quantitative MR methods: Overview and recommendations by the ISMRM quantitative MR study group. Magn Reson Med. 2022;87(3):1184–206.
Hamilton JI, et al. Deep learning reconstruction for cardiac magnetic resonance fingerprinting T1 and T2 mapping. Magn Reson Med. 2021;85(4):2127–35.
Fyrdahl A, Seiberlich N, Hamilton J. Online FIRE reconstruction of cardiac MRF T1, T2 and ECV maps with neural network dictionary generation and low-rank subspace reconstruction. Int Soc Magn Reson Med Annu Meet. London, England. 2022.
Hansen MS, Sørensen TS. Gadgetron: An open source framework for medical image reconstruction. Magn Reson Med. 2013;69(6):1768–76.
Ahad J, et al. Implementation of cardiac MRF in gadgetron for online reconstruction. Proc Annu Meet ISMRM. 2018;4789.
Lo WC, et al. Multicenter repeatability and reproducibility of MR fingerprinting in phantoms and in prostatic tissue. Magn Reson Med. 2022.
Xue H, et al. Distributed MRI reconstruction using Gadgetron-based cloud computing. Magn Reson Med. 2015;73(3):1015–25.
Bustin A, et al. High-resolution free-breathing late gadolinium enhancement cardiovascular magnetic resonance to diagnose myocardial injuries following COVID-19 infection. Eur J Radiol. 2021;144:109960.
Cloos MA, et al. Multiparametric imaging with heterogeneous radiofrequency fields. Nat Commun. 2016;7(1):1–10.
Hong K, et al. Wideband arrhythmia-insensitive-rapid (AIR) pulse sequence for cardiac T1 mapping without image artifacts induced by an implantable-cardioverter-defibrillator. Magn Reson Med. 2015;74(2):336–45.
Acknowledgements
BE acknowledges support from NIH/NIA K25AG070321, Philips Healthcare, and Siemens Healthineers. CP acknowledges support from Millennium Institute ICN2021_004 and Fondecyt 1210637. JH acknowledges support from Michigan Institute for Clinical & Health Research (MICHR) Grant UL1TR002240, Siemens Healthineers, and NIH/NHLBI R01HL163030. NS acknowledges support from grants from Siemens Healthineers, outside the submitted work. In addition, NS has a patent 8,723,518 with royalties paid to Siemens Healthineers.
Funding
W.H. Wilson Tang reports grants from the National Institutes of Health; and personal fees from Sequana Medical, Owkin Inc., preCARDIA, Relypsa Inc. Cardiol Therapeutics, Genomics plc, Zehna Therapeutics LLC, Renovacor Inc., Boston Scientific, WhiteSwell, Springer Nature, and the American Board of Internal Medicine, outside the submitted work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The other authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All images of human subjects as obtained by the authors of this article were acquired under Institutional Review Board approved protocols that include subject consent to participate and consent to publish.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, Philips Healthcare, Siemens Healthineers, Millennium Institute, Fondecyt, or MICHR.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiac PET, CT, and MRI
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eck, B.L., Yim, M., Hamilton, J.I. et al. Cardiac Magnetic Resonance Fingerprinting: Potential Clinical Applications. Curr Cardiol Rep 25, 119–131 (2023). https://doi.org/10.1007/s11886-022-01836-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-022-01836-9