Abstract
Purpose of Review
Following significant advancements in cancer therapeutics and survival, the risk of cancer therapy-related cardiotoxicity (CTRC) is increasingly recognized. With ongoing efforts to reduce cardiovascular morbidity and mortality in cancer patients and survivors, cardiac biomarkers have been studied for both risk stratification and monitoring during and after therapy to detect subclinical disease. This article will review the utility for biomarker use throughout the cancer care continuum.
Recent Findings
A recent meta-analysis shows utility for troponin in monitoring patients at risk for CTRC during cancer therapy. The role for natriuretic peptides is less clear but may be useful in patients receiving proteasome inhibitors. Early studies explore use of myeloperoxidase, growth differentiation factor 15, galectin 3, micro-RNA, and others as novel biomarkers in CTRC.
Summary
Biomarkers have potential to identify subclinical CTRC and may reveal opportunities for early intervention. Further research is needed to elucidate optimal biomarkers and surveillance strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over 18 million patients worldwide were diagnosed with cancer in 2020 [1]. With significant advancements in cancer therapeutics over the last few decades, the prognosis for these patients has improved dramatically. Yet, as patients with cancer live longer, it is increasingly recognized that they have a considerable cardiovascular disease burden with a 2- to 6- fold higher cardiovascular mortality rate than the general population [2]. Cardiovascular disease and cancer often coexist due to their high prevalence, shared risk factors (e.g., smoking), bidirectional relationship (e.g., inflammation), and the potential cardiotoxic effects of cancer treatments. Not only does cardiovascular disease affect the quality of life and survival of patients after cancer treatment, but cardiac events during therapy can lead to discontinuations of treatment and worsen cancer survival.
Since cardiovascular complications are a significant contributor to morbidity and mortality in cancer patients and survivors, several cardiac biomarkers have been evaluated for baseline risk stratification, early recognition of subclinical disease during and after cancer therapy, and prognostication. The ideal cardiac biomarker should be easily measured, reproducible, and reliably predict clinical outcomes. It should also influence management plans by directing either preventive (risk stratification) or treatment (disease detection) regimens. In a seminal paper, Cardinale et al. showed not only that elevated troponin I measured soon after high-dose chemotherapy could detect patients at risk for further left ventricular (LV) dysfunction, but also initiation of enalparil after troponin elevation could prevent LV decline [3]. The efficacy of biomarkers for any given cancer therapy regimen will vary with the mechanism of toxicity and biomarker pathophysiology, though biomarkers can also have more universal applications such as the use of troponin and natriuretic peptides to detect myocardial necrosis/injury and chamber dilation/volume overload, respectively.
This article will review recommendations for biomarker use throughout the cancer care continuum, the underlying pathophysiology of major biomakers, and the current state of the evidence for the utility of biomarkers in risk stratification and disease detection across different cancer types and cancer treatmements. Though cancer therapy-related cardiotoxicity (CTRC) includes a broad range of conditions such as radiation induced valvular disease or atherosclerosis, arrhythmias, and pulmonary hypertension, biomarkers are most commonly used for detection of LV dysfunction and cardiomyopathy. The most investigated and validated biomarkers are troponin and natriuretic peptides (NP), with several emerging biomarkers under study.
Biomarker Utilization at Time of Cancer Diagnosis
At the time of initial diagnosis, baseline biomarker measurement (troponin and NP) can complement a patient’s cardiovascular history and physical examination to help detect those at increased risk of developing cardiac dysfunction or those with pre-existing subclinical disease. These patients may benefit from cardioprotective medications and/or more frequent monitoring during or following cancer treatment. Biomarkers measured at baseline also provide a helpful reference for patients with possible cardiotoxicity after initiating cancer treatment. While acknowledging that further study is needed, the European Society of Medical Oncology (ESMO) recommends considering assessment of baseline biomarkers (troponin, NP) in higher-risk patients (e.g., relapsed myeloma or planned anthracycline-based chemotherapy) to help identify patients at increased risk for LV dysfunction or heart failure (HF) [4]. The European Society of Cardiology (ESC) and International Cardio-Oncology Society (ICOS) have also published a collaborative expert consensus statement unanimously supporting baseline biomarker measurement (troponin, NP) to aid in risk assessment [5]. More recently, the ESC, European Hematology Association, the European Society for Therapeutic Radiology and Oncology, and ICOS published collaborative cardio-oncology guidelines recommending baseline biomarker assessment (troponin, NP) [6••]. Comparisons between consensus recommendations and guidelines are detailed in Table 1.
The best supporting evidence for baseline biomarker assessment for risk assessment or prognostication comes from studies in patients with relapsed myeloma, those receiving anthracycline or anti-human epidermal growth factor receptor 2 (HER2) therapy, and those with amyloidosis. In patients with relapsed myeloma starting carfilzomib therapy, baseline elevation with brain natriuretic peptide (BNP) or N-terminal proBNP (NT-ProBNP) conferred an 11-fold risk for cardiovascular adverse events on treatment [7]. In a study of 452 patients from the Herceptin Adjuvant (HERA) study, baseline troponin elevation conferred a 4.5-fold increased risk for significant LVEF drop following trastuzumab therapy [8]. In patients with AL amyloidosis, biomarker assessment with troponin and NPs are integral to disease staging and survival prognostication as a way to assess for the degree of cardiac involvement [9].
There is no evidence that elevated baseline biomarkers in isolation should influence the decision for, or choice of, cancer treatment. Conversely, baseline risk assessment using biomarkers can be valuable to guide monitoring and screening intervals, cardiovascular risk factor optimization, and potentially identify those most likely to benefit from cardioprotective medications as an adjunct to their cancer treatment. Cancer therapy decisions should always be done in a multidisciplinary fashion (including Hematology/Oncology and Cardiology) with appropriate recognition of the potential survival prolonging benefit of cancer therapy relative to the potential for, or even presence of, cardiovascular toxicity [10].
Biomarker Utilization During Cancer Therapy
During cancer therapy, biomarkers have the potential to identify early subclinical cardiotoxicity before progression to overt cardiac dysfunction. In the aforementioned study by Cardinale and colleagues, elevation of troponin after high-risk chemotherapy identified patients at risk for future LV dysfunction [3]. More importantly, the use of enalapril mitigated subsequent LV dysfunction in patients with elevated troponin. ESMO recommends consideration for routine screening with troponin and NPs every 3–6 weeks during anthracycline treatment [4].
Biomarker assessment is generally recommended for consideration during anthracycline, anti-HER2 therapy, anti-vascular endothelial growth factor (VEGF) therapy, and other potentially cardiotoxic therapies, although data on the optimal screening protocols and patient selection are lacking [4]. There is increased interest for biomarker (troponin, NP) screening during treatment with immune checkpoint inhibitor (ICI) therapy for early detection of myocarditis, an uncommon, but potentially fatal complication of immunotherapy [5].
As in the general population, there is also clinical utility for the use of biomarkers (NP, troponin) as a diagnostic tool in a patient presenting with shortness of breath or other cardiac symptoms during or after cancer treatment. Separately, in patients noted to have a decline in LV function on screening echocardiogram, biomarkers can complement the cardiovascular examination to better quantify the clinical significance of the LV dysfunction [5]. Asymptomatic patients with normal biomarkers are much more likely to tolerate the decline in LV function compared to patients with symptomatic HF or marked increase in NP.
Biomarkers During Cancer Survivorship
Biomarker utilization in cancer survivorship, particularly further out from cancer therapy, has been mainly investigated in adult survivors of childhood cancer. In 535 adults from the St. Jude cohort, 10 or more years out from cancer therapy and without history of cardiomyopathy, NT-ProBNP levels above the age- and sex-specific 97.5th percentile identified patients with a twofold increased risk for subsequent cardiomyopathy [11]. Only five survivors had elevated troponin levels. Theoretically, troponin, a marker of acute cardiac injury, would be less helpful during survivorship, while NPs could identify patients at risk for developing HF, similar to prior data showing utility in identifying subclinical HF in the general population [12]. Based on the significantly increased observed HF incidence, lifelong screening with echocardiogram is recommended by the Children’s Oncology Group for survivors of high-risk cancer therapy (e.g., anthracyclines, chest radiation) with reasonable cost-effectiveness [13]. Biomarkers have not been included in these survivorship recommendations to date. The ESMO guidelines recommend consideration for a CV evaluation that can include biomarkers following cardiotoxic cancer therapy at 6–12 months after therapy, at 2 years, and possibly periodically thereafter [4].
Pathophysiology of Biomarkers
In this section, we briefly review the pathophysiology of established and emerging biomarkers and the mechanisms as they relate to general cardiovascular disease. Specific applications in cardio-oncology are discussed in subsequent sections (Fig. 1).
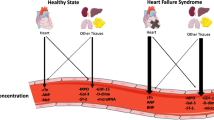
Pathophysiologic mechanisms of biomarkers implicated in cancer therapy related cardiotoxicity. These markers are reflective of myocardial injury (troponin, CK-MB, GDF-15), stretch and stress (BNP), inflammation (hsCRP, IL-6, GDF-15), oxidative stress (MPO, miRNA), fibrosis (MPO, gal-3, miRNA, ST2), and angiogenesis (PlGF). Of these markers, troponin and BNP are most commonly used in cardiac assessment. Whereas troponin is reflective of myocardial damage, BNP is reflective of stress culminating in neurohormonal changes that inhibit the renin-angiotensin system (RAAS) and sympathetic nervous system (SNS). This figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license (https://creativecommons.org/licenses/by/3.0/)
Troponin
Markers of myocardial injury (troponin) were developed to diagnose acute coronary syndromes but have since been shown to have significant value in detecting cardiotoxicity from cancer therapy as well. Troponin acts as the regulatory complex of the cardiac myofibrillar apparatus and is critical in excitation-coupling [14]. While the troponin subunits are generally specific to the cardiac myocyte, emerging literature suggests that troponin T may be present in skeletal muscle as well, and thus, troponin I may be the most precise for cardiac damage [15].
Notably, elevations in troponin are not specific to an underlying clinical mechanism and do not necessarily reflect myocardial necrosis. In response to myocardial stress, troponin can be systemically detected due to transient increases in cell permeability from cellular wounds and cytoplasmic blebbing in addition to apoptosis (Fig. 1) [16]. More recently, the adoption of high-sensitivity cardiac troponin (hs-cTn) allows for the detection of very low troponin concentrations [17]. Thus, clinical scenarios like hypoxemia, anemia, tachyarrhythmias, shock, HF, and kidney disease can cause detectable hs-cTn levels.
Natriuretic Peptides
Distinct to markers of myocardial injury, NPs are neurohormonal modulators that process sodium and water and usually increase with hemodynamic stress and congestion. Of the various NPs, brain natriuretic peptide (BNP) is the most clinically relevant in the diagnosis, treatment, and prognosis of HF as it predicts adverse outcomes at high concentrations [18, 19]. The predominant signal for BNP release is cardiac myocyte stretch in response to elevated intracardiac volume and pressure. It is also transcriptionally upregulated by catecholamines and angiotensin-II [20,21,22,23]. BNP is the biologically active peptide, while the remaining cleaved portion, NT-proBNP, is the inactive segment with a longer half-life [24]. When in its active form, BNP directly inhibits renin–angiotensin–aldosterone system (RAAS) and sympathetic nervous system (SNS) signaling, relaxes vascular smooth muscle cells, and relaxes mesangial cells, preventing renal tubular resorption of sodium. These mechanisms trigger vasodilation, natriuresis, diuresis, a reduction of circulating blood volume, and lower blood pressure (Fig. 1) [23, 25].
While BNP and NT-proBNP are most strongly related to cardiac myocyte stress, there are also emerging data showing a positive correlation between NT-proBNP and inflammatory cytokines like interleukin-6 (IL-6) [26]. In patients with cancer, BNP can be elevated in absence of clinical HF and positively correlates with high-sensitivity C-reactive protein (hsCRP), suggesting a relationship with cancer-related inflammation [27]. BNP can also be markedly elevated in severe sepsis and septic shock [28, 29]. The relationship between BNP, inflammation, and sepsis requires further investigation.
Emerging Biomarkers
Myeloperoxidase
MPO is an inflammatory enzyme produced and secreted by leukocytes in response to myocardial infarction. It generates reactive oxygen species and proteolytic enzymes that promote oxidative damage and extracellular matrix breakdown [30, 31]. This promotes atherogenesis and fibrosis, portraying risk for coronary artery disease (CAD) and HF [32, 33].
Interleukin-6 and High-Sensitivity CRP
IL-6 is a cytokine that not only promotes hepatic production of CRP, but also activates endothelial cells, lymphocyte proliferation and differentiation, coagulation, and the hypothalamic–pituitary–adrenal axis. Both IL-6 and hsCRP are important inflammatory mediators that are cornerstones in the development of coronary artery disease [34]. HsCRP is well established to be predictive of coronary artery disease, MI, stroke, peripheral artery disease, and plaque instability in both primary and secondary prevention studies [35]. Similarly, IL-6 is independently associated with cardiovascular death, MI, all-cause mortality, and risk of hospitalization for HF within a cohort of over 14,000 patients in the STABILITY trial [36].
Interleukin-1 Receptor-Like 1 (ST2)
Also known as Interleukin-1 receptor-like 1 (IL1RL1), ST2 is the receptor of the interleukin-1 (IL-1) family for the ligand IL-33 and is found in two forms, transmembrane and soluble ST2 [37]. Weinberg et al. first showed that ST2 is induced by myocardial stretch and myocardial injury [38]. Where transmembrane ST2 is protective against fibrosis, soluble ST2 sequesters IL-33 and negates these protective effects [37]. Higher levels of soluble ST2 have been shown to predict mortality in both acute and chronic HF [39, 40]. Though soluble ST2 is a relatively weak marker of acute myocardial injury, it is associated with development of HF in non-ST elevation ACS [41].
Galectin-3
Galectin-3 is a member of the lectin family that is released during monocyte differentiation into macrophages and involved in multiple inflammatory signaling mechanisms [42]. It promotes fibroblast proliferation and is pro-fibrotic, inducing cardiac hypertrophy in rat models [43]. Gal-3 is also upregulated in many types of cancer, complicating its utility for detecting cancer therapy-related cardiotoxicity [42]. Gal-3 has shown promising, yet conflicting results as a prognostic marker for all-cause and cardiovascular mortality according to two meta-analyses [44, 45]. An analysis of the Penn Heart Failure Study found that gal-3 significantly correlated with severe HF symptoms and adverse events (all-cause mortality, and risk of cardiac transplantation or VAD placement) in a prospective cohort of chronic HF patients with preserved, reduced, and recovered ejection fraction. Interestingly, gal-3 alone may be a superior marker of adverse events in HF with preserved ejection fraction when compared to those with reduced ejection fraction. This effect is more pronounced when gal-3 is combined with NT-proBNP [44, 46].
Placental Growth Factor
Placental growth factor (PlGF) is a member of the vascular endothelial growth factor family involved in hypertrophy and angiogenesis [47, 48]. It has been shown to be prognostic for all-cause mortality and non-fatal myocardial infarction in acute and long-term follow-up following acute coronary syndromes [49].
Growth Differentiation Factor 15
Growth differentiation factor 15 (GDF-15) is a cytokine of the transforming growth factor-B family. It is released in response to sympathetic activation, ischemia, injury, cardiac remodeling, and inflammation and acts as a protective mechanism against norepinephrine-induced myocardial hypertrophy [50]. Clinically, GDF-15 has been shown to be prognostic for mortality in HF [51].
Micro-RNA
Micro-RNAs (miRNA) are small and ubiquitous, non-coding RNAs that have important regulatory abilities. In the pathogenesis of HF, miRNAs have been involved in myocardial inflammation, apoptosis, hypertrophy, and fibrosis [52]. They have also been reported in acute myocardial infarction, arrhythmias, and pulmonary hypertension [53]. While yielding promise, miRNAs have not yet been found to be reliable biomarkers [54].
Biomarker Assays
When interpreting and comparing biomarker research, it is important to recognize that there is significant variance in biomarker assays and normal reference ranges across different studies and cancer centers. Confounding this issue, traditional troponin assays have more recently been replaced by high-sensitivity assays, allowing for detection of only subtle troponin levels not previously possible in early cardiotoxicity studies. These issues impact generalizability of study findings and must be considered when translating research findings to clinical practice.
Anthracycline-Induced Cardiotoxicity
Anthracyclines such as doxorubicin, daunorubicin, epirubicin, and idarubicin are often the treatment of choice for leukemia, lymphoma, sarcoma, and high-risk breast cancer. As topoisomerase II inhibitors, their cardiotoxic mechanisms include DNA damage, generation of reactive oxygen species, and iron-induced mitochondrial toxicity [55]. The risk of anthracycline-induced HF increases as the cumulative dose administered increases: 3–5% with 400 mg/m2 and as high as 18–48% at 700 mg/m2 [56]. A meta-analysis of patients treated with various doses of anthracyclines across 18 studies reported 6% developed clinical cardiotoxicity and 18% had subclinical cardiotoxicity over a median follow-up of 9 years [57]. Most cardiotoxicity develops in the first year after treatment, although childhood survivors have been noted to be at increased risk for cardiomyopathy throughout their lifetime [58].
Troponin
There is a clear association between anthracycline-associated rise in troponin and future development of LV dysfunction. A 2020 meta-analysis by Michel et al. investigated the utility of troponins and/or NPs across 61 trials with 5691 patients [59••]. Anthracyclines and “high-dose chemotherapy” that included anthracyclines conferred the highest risk for troponin elevation (17.5-fold and 230.4-fold increased risk, respectively) [59••]. Patients with a troponin elevation after anthracycline treatment were subsequently at a sevenfold increased risk for LV dysfunction, with a 54% sensitivity, 79% specificity, and 93% negative predictive value [59••].
Across nine studies of various cancer treatments, preventive therapy with both ACEi/ARB and beta-blockers were associated with reduced troponin elevation on treatment [59••]. ACEi/ARB conferred a numerically stronger protective effect than beta-blockers. Interestingly, metoprolol has generally failed to show a protective benefit during anthracycline treatment, while carvedilol has shown benefit, potentially due to its antioxidant properties [60].
Natriuretic Peptides
Limited by fewer studies, the same 2020 meta-analysis did not find conclusive evidence that elevations in NP levels with anthracycline (or other chemotherapy) could predict LV dysfunction [59••]. Across 4 anthracycline studies, NP levels were higher in patients with LV dysfunction vs those with preserved LVEF (standard mean difference 1.08) [59••]. Only one study of 52 patients evaluated whether an NP cutoff was associated with LV dysfunction, and no statistical difference was found [59••]. By its nature, troponin is a more specific marker of true myocardial injury, which can subsequently lead to future LV dysfunction, while rises in NP can also be associated with therapy associated fluid administration or other inflammatory mechanisms previously discussed. The value of NP may be in its ability to rule out cardiotoxicity, with a BNP < 100 ng/L having a negative predictive value of 92% for anthracycline-induced cardiotoxicity in the PREDICT study of 586 patients [61].
Recommendations
The 2022 ESC guidelines recommend measuring NP and troponin in those at high and very high risk of CTRC at baseline, before every cycle during treatment, and 3 and 12 months following therapy completion. Those at low risk can be monitored at baseline, potentially every two cycles during treatment, and potentially at 3 months following therapy completion (Tables 1 and 2) [6••, 62•].
Emerging Biomarkers
GDF-15, MPO, gal-3, and other novel biomarkers have also been investigated in those receiving anthracycline therapy. In one cohort of patients treated with anthracyclines and trastuzumab, there was an early association between cardiotoxicity, troponin, and MPO [63]. At 15-month follow-up, MPO remained an important predictor of cardiotoxicity, while GDF and PlGF were also associated with cardiotoxicity at this later time point [64]. It is unknown whether these findings can be generalized to anthracycline treatment alone or only to those receiving both anthracyclines and trastuzumab. However, a recent meta-analysis did show that doubling of GDF-15 and gal-3 were associated with an increased risk of anthracycline-induced cardiotoxicity at 3 months (ejection fraction < 50–55% or 10%-point decrease) [65].
Early studies have additionally identified a few microRNA candidates as potential cardiotoxicity biomarkers. MicroRNA146a, MiRNA 140-5p, and MiRNA-377 have all been linked to cardiomyocyte death and/or mortality in animal models of doxorubicin cardiotoxicity [66,67,68], although more conclusive studies are needed regarding pathophysiology, measurement variability, and validation prior to clinical use.
HER2 Directed Therapies
Human epidermal growth factor receptor 2 (HER2) is overexpressed in certain solid tumors, especially breast cancer, promoting proliferation, growth, and survival. HER2 inhibitors (trastuzumab, pertuzumab, lapatinib, neratinib) are the cornerstone for treatment. The cardiotoxic effects of HER2 antagonists are likely due to interruption of HER2 signaling on the cardiomyocyte, whose normal function appears to be integral to healthy cardiac adaptation [69]. LV dysfunction is not dose-dependent and is usually reversible after withdrawal of medication or starting guideline-directed medical therapy for LV dysfunction or HF [70,71,72]. Recent studies have actually shown that patients with mild LV dysfunction (LVEF > 40%) can often be continued safely on treatment with HER2 antagonists [73]. Incidence of HF with trastuzumab monotherapy ranges from 1 to 4% in clinical trials with an incidence of LV systolic dysfunction of 10–15% [5].
Biomarkers in trastuzumab cardiotoxicity have yielded mixed results partly because of concurrent or prior anthracycline therapy, different assays and thresholds, and inconsistent follow-up strategies. The biomarker substudy of the HERA trial measured both troponin and NT-proBNP in 533 women with HER2 + breast cancer (BC) receiving trastuzumab [8]. Though NT-proBNP has higher sensitivity than troponin in detecting new LVD following trastuzumab treatment, neither biomarker reached statistical significance [8]. Cardinale et al. showed that TnI elevation can be an important marker in identifying those who will develop HER-2 therapy cardiotoxicity and who are less likely to recover LVEF with HF treatment [74]. Importantly, most initial TnI elevations occurred after cycle 1 of treatment [74]. An observational study of 66 patients receiving trastuzumab found that NT-proBNP was significant associated with developing cardiotoxicity [75]. Other studies suggest hs-CRP may predict HER2 cardiotoxicity [76].
Given the lack of consistent data, there are no specific guidelines for cardiac monitoring during HER2-therapy in asymptomatic patients. The 2022 ESC guidelines recommend baseline measurement of NP and troponin in those receiving HER2 therapy to help identify those at increased risk during treatment, with consideration for monitoring while on therapy (Table 1) [6••]. Biomarkers continue to be investigated as a potential adjunct to regular imaging and may play an important role in patients with reduced access to imaging such as in the novel coronavirus-19 pandemic [77].
Immune Checkpoint Inhibitors
ICI (e.g., pembrolizumab, ipilimumab, nivolumab) are a quickly growing class of immunomodulatory treatments that promote T-cell mediated destruction of tumor cells by inhibiting various binding sites (e.g., PD-1, PD-L1, and CTLA-4). ICI have been linked to accelerated atherosclerosis and associated CV events [78], but the most concerning cardiotoxicity is myocarditis, an uncommon but potentially fatal complication associated with activation of the immune system. Since myocarditis can respond to treatment, especially if found early, there is interest in developing screening algorithms incorporating troponin levels. In a multi-center registry of patients with ICI-associated myocarditis, 94% of patients had elevated troponin levels, although selection bias is a concern in registry-based research [79]. An elevation of troponin greater than the 99th percentile of the upper limit of normal is included as part of the minor criteria for the diagnosis of ICI-associated myocarditis [80].
The 2022 ESC guidelines recommend baseline troponin and NP assessment prior to ICI treatment and consideration for troponin measurements before doses 2, 3, and 4 and then every 3 doses, thereafter (Table 1) [6••]. While many organizations agree on baseline assessment of cardiac biomarkers for reference during treatment and risk assessment, the utility of troponin surveillance in asymptomatic patients during ICI treatment is less clear, and no consensus has been reached [81]. Troponin cannot differentiate ICI-induced myocarditis from other much more common causes of myocardial injury. Its measurement can potentially facilitate early diagnosis and prompt early intervention of serious adverse cardiac events including ICI-associated myocarditis. Importantly, there is no evidence that cancer therapy decisions should be based on a troponin elevation alone without any other evidence of clinically significant disease, especially given the tremendous life-prolonging impact that immunotherapy can exert on several cancer types.
Multiple Myeloma and Proteasome Inhibitors
A foundational treatment of multiple myeloma, proteasome inhibitors (PI) lead to apoptosis by preventing degradation of intracellular proteins. PIs such as Ixazomib and bortezomib act as reversible inhibitors while carfilzomib is an irreversible selective inhibitor [82]. PIs have been associated with LVSD/HF in the first 3 months of therapy, particularly carfilzomib [7]. In a cohort of 95 patients with relapsed multiple myeloma receiving bortezomib or carfilzomib, elevation in NPs were common and predicted subsequent cardiovascular adverse events (symptomatic HF, acute coronary syndrome, symptomatic arrhythmia, venous thromboembolic events, pulmonary hypertension, grade 3 hypertension, or cardiac chest pain requiring treatment) [7]. Subsequently, the 2020 HFA and ESC position statement recommends considering NP measurement at baseline and during the first few cycles, particularly if the patient is being treated with carfilzomib [5].
Cardiac Amyloidosis
Related to multiple myeloma, AL amyloid has a propensity for cardiac involvement, and baseline troponin and NP assessment have long been cornerstones for staging and prognosis [83]. More recently, it has also been noted that a 30% reduction in NP with AL treatment is associated with better survival and clinical outcomes, though the prognostic value of NT-proBNP is less certain in those with an eGFR < 15 mL/min/1.7m2 [9, 84,85,86]. Thus, NP can also be used to help track response to treatment. Current criteria in assessing AL amyloidosis response to therapy are based on light chain, creatinine, and NT-proBNP level, and current guidelines recommend biomarker assessment every 3–6 months [87, 88].
Carcinoid Heart Disease
Carcinoid syndrome is a rare endocrine neoplasm that arises mostly from enterochromaffin cells and has it effects mediated by vasoactive substances like serotonin [89]. Patients with carcinoid syndrome can have tricuspid and pulmonic valve involvement (20–50%) with rare (< 10%) left-sided valve involvement as the vasoactive substances are deactivated in the lungs [90]. Among patients with carcinoid syndrome, a NT-proBNP level cutoff of 250 pg/mL had a 92% sensitivity and 91% specificity for carcinoid heart disease (CHD) [91]. Aside from predicting CHD, NT-proBNP also correlates well with survival [92]. Chromogranin A (CgA) has been found to be sensitive for CHD, but with low specificity [92]. Given current evidence, NT-proBNP level and echocardiography are recommended to screen for CHD in patients with carcinoid syndrome even in the absence of symptoms or signs of valvular heart disease [93, 94].
Radiation-Induced Cardiovascular Disease
Over half of patients with thoracic cancer will be treated with radiation therapy (RT), which can result in incidental radiation to cardiac tissue. Consequently, an estimated one-third of thoracic cancer patients undergoing RT will develop radiation-induced cardiovascular disease (RICVD), which can include coronary artery disease, conduction abnormalities, myocardial fibrosis, peripheral vascular disease, and pericarditis [95]. The underlying pathophysiology of RICVD involves both direct damage to nucleic acids and biomolecules and indirect cellular injury via generation of reactive oxygen species, danger-associated molecular patterns, and other inflammatory factors [96]. While cardiomyocytes are relatively radioresistant, coronary endothelial cells are particularly sensitive to radiation injury, and therefore, coronary endothelial dysfunction is believed to be a critical initial step in RICVD [97]. Despite early radiation-induced cardiac changes, overt RICVD may not be diagnosed in patients until years after RT. Therefore, identification of RICVD biomarkers is a critical step in improving early detection, surveillance, and treatment of cardiac dysfunction in thoracic cancer patients treated with RT.
Several conventional markers of cardiac damage have been investigated in the context of RICVD. In female patients with left-sided breast cancer, troponin can be elevated within circulation immediately and 3 months post-RT [71, 98, 99], with troponin-I levels at 3 months post-RT correlating with mean radiation heart dose [98]. Similarly, BNP and NT-proBNP also can increase within 1 year of RT in breast cancer patients and vary with cardiac radiation dose [100, 101]. However, troponin and NPs elevations post-RT have not been consistently correlated with cardiac outcomes to date.
Beyond these conventional cardiac markers, there are several emerging biomarkers of RICVD under investigation. Emerging “omics” (e.g., genomics, metabolomics) tools hold potential for identifying new RICVD biomarkers, as alterations in nucleic acids and cellular metabolism are among the first to occur with radiation deposition [102, 103]. Other emerging biomarkers being applied to RICVD surveillance include lipopolysaccharide binding protein, which has been found to positively correlate with cardiac dose and post-RT diastolic function, gal-3, and pro-inflammatory cytokines (TNF-a, IL-1, IL-6) [71, 103,104,105]. Increased ST2 levels following adjuvant radiotherapy in chemo-naïve breast cancer patients have also correlated to worsening GLS; however, clinical outcomes are unknown [106]. Combining multiple plasma markers, or alternatively, a plasma biomarker with echocardiographic parameters such as global longitudinal strain or LV function, may improve RICVD prediction [63, 107]. There are no specific recommended screening guidelines for using biomarkers to detect RICVD at this time.
VEGF Inhibitors
Vascular endothelial growth factor (VEGF) inhibitors prevent activation of cellular processes promoting angiogenesis and are commonly used in the treatment for renal cell carcinoma as well as metastatic colon and gastric cancer [108]. Hypertension is a common CV side effect of VEGF inhibitors. The resultant increase in afterload and simultaneous use of other cardiotoxic anti-cancer therapies puts patients at higher risk of LV dysfunction [5, 109]. In a study of 159 patients with renal cell carcinoma treated with VEGF inhibitors, NP were associated with LV dysfunction [110]. There is currently no data to support troponin monitoring in patients treated with VEGF inhibitors as it was unable to predict LVSD, HF, or ischemic events in one small study [110]. NP on the other hand may precede LVEF reduction or clinically relevant HF in cancer patients treated with anti-VEGF therapy [5, 110]. Current expert recommendations include blood pressure monitoring at every clinic visit with NP assessment at baseline, 1 month following treatment, and every 3 months thereafter [5]. The 2022 ESC guidelines recommend NP and troponin measurement at baseline and every 4 months in the first year of treatment in moderate-risk patients, and at baseline, 4 weeks, and every 3 months in the first year of treatment in high and very-high risk patients (Tables 1 and 2).
Conclusions
Cardiac biomarkers, primarily troponin and NP, have become integral in assessing baseline risk and monitoring for subclinical cardiotoxicity in patients during and after treatment with high-risk cancer therapy. Based on available evidence, biomarkers are strongly featured in recommendations for management of patients on anthracyclines, anti-HER2 antagonists, immunotherapy, proteasome inhibitors, and VEGF inhibitors as well as patients being evaluated for amyloidosis. Nevertheless, many questions remain regarding optimal protocols and prognostic algorithms in various types of cancer and cancer therapeutics. Numerous novel biomarkers are also emerging with promise in the world of cardio-oncology such as MPO and micro-RNAs. Further research is needed to better elucidate their clinical utility to predict cardiovascular events and refine prognosis in patients with cancer or a history of cancer.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Sturgeon KM, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40(48):3889–97.
Cardinale D, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114(23):2474–81.
Curigliano G, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31(2):171–90.
Pudil R, et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: a position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur J Heart Fail. 2020;22(11):1966–83.
•• Lyon AR, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022. These are the first published cardio-oncology guidelines. Recommendations include pre-treatment cardiovascular assessment, surveillance during cancer therapy, and monitoring during survivorship.
Cornell RF, et al. Prospective study of cardiac events during proteasome inhibitor therapy for relapsed multiple myeloma. J Clin Oncol. 2019;37(22):1946–55.
Zardavas D, et al. Role of troponins I and T and N-terminal prohormone of brain natriuretic peptide in monitoring cardiac safety of patients with early-stage human epidermal growth factor receptor 2-positive breast cancer receiving trastuzumab: a herceptin adjuvant study cardiac marker substudy. J Clin Oncol. 2017;35(8):878–84.
Pregenzer-Wenzler A, et al. Utility of biomarkers in cardiac amyloidosis. JACC Heart Fail. 2020;8(9):701–11.
Lenihan DJ, Zhang K, Mitchell J. The washington manual of cardio-oncology: a practical guide for improved cancer survivorship. 2022: Lippincott Williams & Wilkins.
Dixon SB, et al. Cardiac biomarkers and association with subsequent cardiomyopathy and mortality among adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort. Cancer. 2020.
Natriuretic Peptides Studies Collaboration, et al. Natriuretic peptides and integrated risk assessment for cardiovascular disease: an individual-participant-data meta-analysis. Lancet Diabetes Endocrinol. 2016;4(10):840–9.
Wong FL, et al. Cost-effectiveness of the children’s oncology group long-term follow-up screening guidelines for childhood cancer survivors at risk for treatment-related heart failure. Ann Intern Med. 2014;160(10):672–83.
Parmacek MS, Solaro RJ. Biology of the troponin complex in cardiac myocytes. Prog Cardiovasc Dis. 2004;47(3):159–76.
Thygesen K, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72(18):2231–64.
Mair J, et al. How is cardiac troponin released from injured myocardium? Eur Heart J Acute Cardiovasc Care. 2018;7(6):553–60.
Januzzi JL Jr, et al. Recommendations for institutions transitioning to high-sensitivity troponin testing: JACC Scientific Expert Panel. J Am Coll Cardiol. 2019;73(9):1059–77.
Januzzi JL Jr, et al. Utility of amino-terminal pro-brain natriuretic peptide testing for prediction of 1-year mortality in patients with dyspnea treated in the emergency department. Arch Intern Med. 2006;166(3):315–20.
Masson S, et al. Prognostic value of changes in N-terminal pro-brain natriuretic peptide in Val-HeFT (valsartan heart failure trial). J Am Coll Cardiol. 2008;52(12):997–1003.
Magga J, et al. B-type natriuretic peptide: a myocyte-specific marker for characterizing load-induced alterations in cardiac gene expression. Ann Med. 1998;30(Suppl 1):39–45.
Tokola H, et al. Mechanical load-induced alterations in B-type natriuretic peptide gene expression. Can J Physiol Pharmacol. 2001;79(8):646–53.
Harada M, et al. Interaction of myocytes and nonmyocytes is necessary for mechanical stretch to induce ANP/BNP production in cardiocyte culture. J Cardiovasc Pharmacol. 1998;31(Suppl 1):S357–9.
Okamoto R, et al. BNP as a major player in the heart-kidney connection. Int J Mol Sci. 2019;20(14).
Hall C. NT-ProBNP: the mechanism behind the marker. J Card Fail. 2005;11(5 Suppl):S81–3.
Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339(5):321–8.
Fish-Trotter H, et al. Inflammation and circulating natriuretic peptide levels. Circ Heart Fail. 2020;13(7):e006570.
Bando S, et al. Plasma brain natriuretic peptide (BNP) level is elevated in patients with cancer. Eur Heart J. 2013;34(suppl_1).
Witthaut R, et al. Plasma atrial natriuretic peptide and brain natriuretic peptide are increased in septic shock: impact of interleukin-6 and sepsis-associated left ventricular dysfunction. Intensive Care Med. 2003;29(10):1696–702.
Ueda S, et al. Prognostic value of increased plasma levels of brain natriuretic peptide in patients with septic shock. Shock. 2006;26(2):134–9.
Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25(6):1102–11.
Fu X, et al. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem. 2001;276(44):41279–87.
Ananthan K, Lyon AR. The role of biomarkers in cardio-oncology. J Cardiovasc Transl Res. 2020;13(3):431–50.
Janus SE, et al. Myeloperoxidase is independently associated with incident heart failure in patients with coronary artery disease and kidney disease. Curr Probl Cardiol. 2021: 101080.
Hartman J, Frishman WH. Inflammation and atherosclerosis: a review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol Rev. 2014;22(3):147–51.
Silva D, Pais de Lacerda A. High-sensitivity C-reactive protein as a biomarker of risk in coronary artery disease. Rev Port Cardiol. (English edition).
Held C, et al. Inflammatory biomarkers interleukin-6 and C-reactive protein and outcomes in stable coronary heart disease: experiences from the STABILITY (stabilization of atherosclerotic plaque by initiation of darapladib therapy) Trial. J Am Heart Assoc. 2017;6(10).
Pascual-Figal DA, Januzzi JL. The biology of ST2: the international ST2 consensus panel. Am J Cardiol. 2015;115(7 Suppl):3b–7b.
Weinberg EO, et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106(23):2961–6.
Januzzi JL Jr, et al. Measurement of the interleukin family member ST2 in patients with acute dyspnea: results from the PRIDE (pro-brain natriuretic peptide investigation of dyspnea in the emergency department) study. J Am Coll Cardiol. 2007;50(7):607–13.
Ky B, et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4(2):180–7.
Kohli P, et al. Role of ST2 in non–ST-elevation acute coronary syndrome in the MERLIN-TIMI 36 Trial. Clin Chem. 2012;58(1):257–66.
Dong R, et al. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (review). Int J Mol Med. 2018;41(2):599–614.
Liu YH, et al. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am J Physiol Heart Circ Physiol. 2009;296(2):H404–12.
Srivatsan V, George M, Shanmugam E. Utility of galectin-3 as a prognostic biomarker in heart failure: where do we stand? Eur J Prev Cardiol. 2015;22(9):1096–110.
Chen A, et al. Prognostic value of serum galectin-3 in patients with heart failure: a meta-analysis. Int J Cardiol. 2015;182:168–70.
French B, et al. Prognostic value of galectin-3 for adverse outcomes in chronic heart failure. J Card Fail. 2016;22(4):256–62.
Russell KS, et al. Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis. Am J Physiol. 1999;277(6):H2205–11.
Accornero F, et al. Placental growth factor regulates cardiac adaptation and hypertrophy through a paracrine mechanism. Circ Res. 2011;109(3):272–80.
Lenderink T, et al. Elevated placental growth factor levels are associated with adverse outcomes at four-year follow-up in patients with acute coronary syndromes. J Am Coll Cardiol. 2006;47(2):307–11.
Xu XY, et al. Growth differentiation factor (GDF)-15 blocks norepinephrine-induced myocardial hypertrophy via a novel pathway involving inhibition of epidermal growth factor receptor transactivation. J Biol Chem. 2014;289(14):10084–94.
Kempf T, et al. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol. 2007;50(11):1054–60.
Huang YM, et al. The diagnostic value of circulating microRNAs in heart failure. Exp Ther Med. 2019;17(3):1985–2003.
Zhou SS, et al. miRNAS in cardiovascular diseases: potential biomarkers, therapeutic targets and challenges. Acta Pharmacol Sin. 2018;39(7):1073–84.
Peterlin A, et al. The role of microRNAs in heart failure: a systematic review. Front Cardiovasc Med. 2020;7:161.
Zhang S, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18(11):1639–42.
Curigliano G, et al. Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J Clin. 2016;66(4):309–25.
Lotrionte M, et al. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am J Cardiol. 2013;112(12):1980–4.
Cardinale D, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131(22):1981–8.
•• Michel L, et al. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: a meta-analysis. Eur J Heart Fail. 2020;22(2):350–61. In this large meta-analysis, findings show that rise in troponin confers the highest risk for developing LV dysfunction in patients treated with anthracyclines and high-dose chemotherapy.
Huang S, et al. Protective role of beta-blockers in chemotherapy-induced cardiotoxicity-a systematic review and meta-analysis of carvedilol. Heart Fail Rev. 2019;24(3):325–33.
Stevens PL, et al. Prediction and early detection of anthracycline-related cardiotoxicity using cardiac biomarkers. J Clin Oncol. 2014;32(15_suppl):9644–9644.
• Lyon AR, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail. 2020;22(11):1945–60. This position statement includes a concensus method for baseline cardiovascular risk assessment for patients with planned cancer therapy.
Ky B, et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63(8):809–16.
Putt M, et al. Longitudinal changes in multiple biomarkers are associated with cardiotoxicity in breast cancer patients treated with doxorubicin, taxanes, and trastuzumab. Clin Chem. 2015;61(9):1164–72.
Kastora SL, et al. Biomarker determinants of early anthracycline-induced left ventricular dysfunction in breast cancer: a systematic review and meta-analysis. Mol Diagn Ther. 2022;26(4):369–82.
Horie T, et al. Acute doxorubicin cardiotoxicity is associated with miR-146a-induced inhibition of the neuregulin-ErbB pathway. Cardiovasc Res. 2010;87(4):656–64.
Zhao L, et al. MicroRNA-140-5p aggravates doxorubicin-induced cardiotoxicity by promoting myocardial oxidative stress via targeting Nrf2 and Sirt2. Redox Biol. 2018;15:284–96.
Henderson J, et al. microRNA-377 signaling modulates anticancer drug-induced cardiotoxicity in mice. Front Cardiovasc Med. 2021;8: 737826.
Vermeulen Z, Segers VF, De Keulenaer GW. ErbB2 signaling at the crossing between heart failure and cancer. Basic Res Cardiol. 2016;111(6):60.
Vallabhaneni S, Krone R, Zhang K. “Cardiac Dysfunction: Traditional chemotherapy, human epidermal growth factor receptor 2 based therapy and radiation” in Lenihan DL, Mitchell J, Zhang K Washington Manual of Cardio Oncology. Wolter Kluwers; 2023:25–47.
Xiao H, et al. Advances in biomarkers for detecting early cancer treatment-related cardiac dysfunction. Front Cardiovasc Med. 2021;8:753313.
Henri C, Heinonen T, Tardif JC. The role of biomarkers in decreasing risk of cardiac toxicity after cancer therapy. Biomark Cancer. 2016;8(Suppl 2):39–45.
Lynce F, et al. Prospective evaluation of the cardiac safety of HER2-targeted therapies in patients with HER2-positive breast cancer and compromised heart function: the SAFE-HEaRt study. Breast Cancer Res Treat. 2019;175(3):595–603.
Cardinale D, et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28(25):3910–6.
Blancas I, et al. NT-proBNP as predictor factor of cardiotoxicity during trastuzumab treatment in breast cancer patients. Breast. 2020;54:106–13.
Onitilo AA, et al. High-sensitivity C-reactive protein (hs-CRP) as a biomarker for trastuzumab-induced cardiotoxicity in HER2-positive early-stage breast cancer: a pilot study. Breast Cancer Res Treat. 2012;134(1):291–8.
Lenihan D, et al. Cardio-oncology care in the era of the coronavirus disease 2019 (COVID-19) pandemic: an International Cardio-Oncology Society (ICOS) statement. CA Cancer J Clin. 2020;70(6):480–504.
Drobni ZD, et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation. 2020;142(24):2299–311.
Mahmood SS, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755–64.
Bonaca MP, et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation. 2019;140(2):80–91.
Delombaerde D, et al. Clinical implications of isolated troponinemia following immune checkpoint inhibitor therapy. ESMO Open. 2021;6(4):100216.
Armenian SH, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35(8):893–911.
Kumar S, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30(9):989–95.
Palladini G, et al. Circulating amyloidogenic free light chains and serum N-terminal natriuretic peptide type B decrease simultaneously in association with improvement of survival in AL. Blood. 2006;107(10):3854–8.
Palladini G, et al. The combination of high-sensitivity cardiac troponin T (hs-cTnT) at presentation and changes in N-terminal natriuretic peptide type B (NT-proBNP) after chemotherapy best predicts survival in AL amyloidosis. Blood. 2010;116(18):3426–30.
Palladini G, et al. Best use of cardiac biomarkers in patients with AL amyloidosis and renal failure. Am J Hematol. 2012;87(5):465–71.
Wechalekar AD, et al. Guidelines on the management of AL amyloidosis. Br J Haematol. 2015;168(2):186–206.
Garcia-Pavia P, et al. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2021;42(16):1554–68.
Jin C, et al. Carcinoid heart disease: pathophysiology, pathology, clinical manifestations, and management. Cardiology. 2021;146(1):65–73.
Bober B, et al. Carcinoid heart disease: how to diagnose and treat in 2020? Clin Med Insights Cardiol. 2020;14:1179546820968101.
Bhattacharyya S, et al. Usefulness of N-terminal pro-brain natriuretic peptide as a biomarker of the presence of carcinoid heart disease. Am J Cardiol. 2008;102(7):938–42.
Korse CM, et al. Chromogranin-A and N-terminal pro-brain natriuretic peptide: an excellent pair of biomarkers for diagnostics in patients with neuroendocrine tumor. J Clin Oncol. 2009;27(26):4293–9.
Davar J, et al. Diagnosing and managing carcinoid heart disease in patients with neuroendocrine tumors: an expert statement. J Am Coll Cardiol. 2017;69(10):1288–304.
Kaltsas G, et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: pre- and perioperative therapy in patients with neuroendocrine tumors. Neuroendocrinology. 2017;105(3):245–54.
Wang H, et al. Radiation-induced heart disease: a review of classification, mechanism and prevention. Int J Biol Sci. 2019;15(10):2128–38.
Mitchell JD, et al. Cardiovascular manifestations resulting from therapeutic radiation: a multidisciplinary statement from the International Cardio-Oncology Society. JACC: CardioOncology. 2021:3(3).
Venkatesulu BP, et al. Radiation-induced endothelial vascular injury: a review of possible mechanisms. JACC Basic Transl Sci. 2018;3(4):563–72.
Speers C, et al. Cardiac magnetic resonance imaging and blood biomarkers for evaluation of radiation-induced cardiotoxicity in patients with breast cancer: results of a phase 2 clinical trial. Int J Radiat Oncol Biol Phys. 2022;112(2):417–25.
Erven K, et al. Subclinical cardiotoxicity detected by strain rate imaging up to 14 months after breast radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(5):1172–8.
Upshaw JN. The role of biomarkers to evaluate cardiotoxicity. Curr Treat Options Oncol. 2020;21(10):79.
D’Errico MP, et al. Kinetics of B-type natriuretic peptide plasma levels in patients with left-sided breast cancer treated with radiation therapy: Results after one-year follow-up. Int J Radiat Biol. 2015;91(10):804–9.
Gramatyka M, Sokol M. Radiation metabolomics in the quest of cardiotoxicity biomarkers: the review. Int J Radiat Biol. 2020;96(3):349–59.
Pang L, et al. Improving cardiotoxicity prediction in cancer treatment: integration of conventional circulating biomarkers and novel exploratory tools. Arch Toxicol. 2021;95(3):791–805.
Dreyfuss AD, et al. A novel mouse model of radiation-induced cardiac injury reveals biological and radiological biomarkers of cardiac dysfunction with potential clinical relevance. Clin Cancer Res. 2021;27(8):2266–76.
Azimzadeh O, et al. Data-Independent acquisition proteomics reveals long-term biomarkers in the serum of C57BL/6J mice following local high-dose heart irradiation. Front Public Health. 2021;9:678856.
Aula H, et al. ST2 levels increased and were associated with changes in left ventricular systolic function during a three-year follow-up after adjuvant radiotherapy for breast cancer. Breast. 2020;49:183–6.
Jacobse JN, et al. Myocardial dysfunction in long-term breast cancer survivors treated at ages 40–50 years. Eur J Heart Fail. 2020;22(2):338–46.
Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176(6):1248–64.
Jimenez J, Alvarez Cardona J. "Cardiac Dysfunction: Small molecule kinase inhibitors, immune based therapies and proteosome inhibitors" in Lenihan DL, Mitchell J, Zhang K Washington Manual of Cardio Oncology. Wolter Kluwers; 2023:48–63.
Hall PS, et al. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Fail. 2013;1(1):72–8.
Funding
This manuscript is in part supported by National Institute of Health award number R01HL147884.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Mitchell reports grants from Pfizer, Abbott Laboratories, Myocardial Solutions, and Children’s Discovery Institute. He has received modest consulting fees from Pfizer and BridgeBio, unrelated to the contents of the manuscript. All other authors report no disclosures.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardio-Oncology
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Joolharzadeh, P., Rodriguez, M., Zaghlol, R. et al. Recent Advances in Serum Biomarkers for Risk Stratification and Patient Management in Cardio-Oncology. Curr Cardiol Rep 25, 133–146 (2023). https://doi.org/10.1007/s11886-022-01834-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-022-01834-x