Abstract
Purpose of the Review
Primary aldosteronism (PA) is the leading cause of secondary hypertension, accounting for over 10% of patients with high blood pressure. It is characterized by autonomous production of aldosterone from the adrenal glands leading to low-renin levels. The two most common forms arise from bilateral adrenocortical hyperplasia (BAH) and aldosterone-producing adenoma (APA). We discuss recent discoveries in the genetics of PA.
Recent Findings
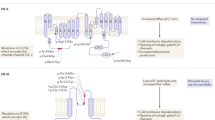
Most APAs harbor variants in the KCNJ5, CACNA1D, ATP1A1, ATP2B3, and CTNNB1 genes. With the exception of β-catenin (CTNNB1), all other causative genes encode ion channels; pathogenic variants found in PA lead to altered ion transportation, cell membrane depolarization, and consequently aldosterone overproduction. Some of these genes are found mutated in the germline state (CYP11B2, CLCN2, KCNJ5, CACNA1H, and CACNA1D), leading then to familial hyperaldosteronism, and often BAH rather than single APAs.
Summary
Several genetic defects in the germline or somatic state have been identified in PA. Understanding how these molecular abnormalities lead to excess aldosterone contributes significantly to the elucidation of the pathophysiology of low-renin hypertension. It may also lead to new and more effective therapies for this disease acting at the molecular level.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lim SS, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–60. https://doi.org/10.1016/S0140-6736(12)61766-8.
Brown JM, et al. The Unrecognized Prevalence of Primary Aldosteronism: A Cross-sectional Study. Ann Intern Med. 2020;173(1):10–20. https://doi.org/10.7326/M20-0065.
Monticone S, et al. Prevalence and Clinical Manifestations of Primary Aldosteronism Encountered in Primary Care Practice. J Am Coll Cardiol. 2017;69(14):1811–20. https://doi.org/10.1016/j.jacc.2017.01.052.
El-Asmar N, Rajpal A, Arafah BM. Primary Hyperaldosteronism: Approach to Diagnosis and Management. Med Clin North Am. 2021;105(6):1065–80. https://doi.org/10.1016/j.mcna.2021.06.007.
Rossi GP, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48(11):2293–300. https://doi.org/10.1016/j.jacc.2006.07.059.
Fountain JH, Lappin SL. Physiology, Renin Angiotensin System. In StatPearls. Treasure Island (FL). 2021. https://www.ncbi.nlm.nih.gov/books/NBK470410/. Accessed 6 Jun 2022
Seidel E, Schewe J, Scholl UI. Genetic causes of primary aldosteronism. Exp Mol Med. 2019;51(11):1–12. https://doi.org/10.1038/s12276-019-0337-9.
Spat A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev. 2004;84(2):489–539. https://doi.org/10.1152/physrev.00030.2003.
Funder JW, et al. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(5):1889–1916. https://doi.org/10.1210/jc.2015-4061.
Conn JW. Presidential address. I. Painting background. II. Primary aldosteronism, a new clinical syndrome. J Lab Clin Med. 1955;45(1):3–17.
Milliez P, et al. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45(8):1243–8. https://doi.org/10.1016/j.jacc.2005.01.015.
Mulatero P, et al. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98(12):4826–33. https://doi.org/10.1210/jc.2013-2805.
Funder JW, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(9):3266–3281. https://doi.org/10.1210/jc.2008-0104.
Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf). 2007;66(5):607–18. https://doi.org/10.1111/j.1365-2265.2007.02775.x.
Kamilaris CDC, Stratakis CA, Hannah-Shmouni F. Molecular Genetic and Genomic Alterations in Cushing’s Syndrome and Primary Aldosteronism. Front Endocrinol (Lausanne). 2021;12: 632543. https://doi.org/10.3389/fendo.2021.632543.
Scholl UI, et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet. 2013;45(9):1050–4. https://doi.org/10.1038/ng.2695.
Beuschlein F, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45(4):440–4, 444e1–2. https://doi.org/10.1038/ng.2550.
Azizan EA, et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet. 2013;45(9):1055–60. https://doi.org/10.1038/ng.2716.
Fernandes-Rosa FL, et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension. 2014;64(2):354–61. https://doi.org/10.1161/HYPERTENSIONAHA.114.03419.
Fernandes-Rosa FL, et al. A gain-of-function mutation in the CLCN2 chloride channel gene causes primary aldosteronism. Nat Genet. 2018;50(3):355–61. https://doi.org/10.1038/s41588-018-0053-8.
Byrd JB, Turcu AF, Auchus RJ. Primary Aldosteronism: Practical Approach to Diagnosis and Management. Circulation. 2018;138(8):823–35. https://doi.org/10.1161/CIRCULATIONAHA.118.033597.
Tan GC, et al. Aldosterone-Producing Adenomas: Histopathology-Genotype Correlation and Identification of a Novel CACNA1D Mutation. Hypertension. 2017;70(1):129–36. https://doi.org/10.1161/HYPERTENSIONAHA.117.09057.
Williams TA, et al. Somatic ATP1A1, ATP2B3, and KCNJ5 mutations in aldosterone-producing adenomas. Hypertension. 2014;63(1):188–95. https://doi.org/10.1161/HYPERTENSIONAHA.113.01733.
Nanba K, et al. Somatic CACNA1H Mutation As a Cause of Aldosterone-Producing Adenoma. Hypertension. 2020;75(3):645–9. https://doi.org/10.1161/HYPERTENSIONAHA.119.14349.
Choi M, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331(6018):768–72. https://doi.org/10.1126/science.1198785.
Akerstrom T, et al. Comprehensive re-sequencing of adrenal aldosterone producing lesions reveal three somatic mutations near the KCNJ5 potassium channel selectivity filter. PLoS ONE. 2012;7(7): e41926. https://doi.org/10.1371/journal.pone.0041926.
Boulkroun S, et al. Prevalence, clinical, and molecular correlates of KCNJ5 mutations in primary aldosteronism. Hypertension. 2012;59(3):592–8. https://doi.org/10.1161/HYPERTENSIONAHA.111.186478.
Lenzini L, et al. A Meta-Analysis of Somatic KCNJ5 K(+) Channel Mutations In 1636 Patients With an Aldosterone-Producing Adenoma. J Clin Endocrinol Metab. 2015;100(8):E1089–95. https://doi.org/10.1210/jc.2015-2149.
Rossi GP, et al. KCNJ5 gene somatic mutations affect cardiac remodelling but do not preclude cure of high blood pressure and regression of left ventricular hypertrophy in primary aldosteronism. J Hypertens. 2014;32(7):1514–21; discussion 1522. https://doi.org/10.1097/HJH.0000000000000186.
Kitamoto T, et al. Comparison of cardiovascular complications in patients with and without KCNJ5 gene mutations harboring aldosterone-producing adenomas. J Atheroscler Thromb. 2015;22(2):191–200. https://doi.org/10.5551/jat.24455.
• Chang YY, et al. KCNJ5 Somatic Mutations in Aldosterone-Producing Adenoma Are Associated With a Worse Baseline Status and Better Recovery of Left Ventricular Remodeling and Diastolic Function. Hypertension. 2021;77(1):114–125. https://doi.org/10.1161/HYPERTENSIONAHA.120.15679. The findings of this study are important considerations in the management of patients with aldosterone-producing adenomas.
Kitamoto T, et al. KCNJ5 mutation as a predictor for resolution of hypertension after surgical treatment of aldosterone-producing adenoma. J Hypertens. 2018;36(3):619–27. https://doi.org/10.1097/HJH.0000000000001578.
Stindl J, et al. Pathogenesis of Adrenal Aldosterone-Producing Adenomas Carrying Mutations of the Na(+)/K(+)-ATPase. Endocrinology. 2015;156(12):4582–91. https://doi.org/10.1210/en.2015-1466.
Tauber P, et al. Cellular Pathophysiology of an Adrenal Adenoma-Associated Mutant of the Plasma Membrane Ca(2+)-ATPase ATP2B3. Endocrinology. 2016;157(6):2489–99. https://doi.org/10.1210/en.2015-2029.
Nanba K, et al. Targeted Molecular Characterization of Aldosterone-Producing Adenomas in White Americans. J Clin Endocrinol Metab. 2018;103(10):3869–76. https://doi.org/10.1210/jc.2018-01004.
Scholl UI, et al. Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype. Clin Endocrinol (Oxf). 2015;83(6):779–89. https://doi.org/10.1111/cen.12873.
Nanba K, et al. Prevalence of Somatic Mutations in Aldosterone-Producing Adenomas in Japanese Patients. J Clin Endocrinol Metab. 2020;105(11). https://doi.org/10.1210/clinem/dgaa595.
Omata K, et al. Cellular and Genetic Causes of Idiopathic Hyperaldosteronism. Hypertension. 2018;72(4):874–80. https://doi.org/10.1161/HYPERTENSIONAHA.118.11086.
Scholl UI, et al. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. Elife. 2015;4:e06315. https://doi.org/10.7554/eLife.06315.
Scholl UI, et al. CLCN2 chloride channel mutations in familial hyperaldosteronism type II. Nat Genet. 2018;50(3):349–54. https://doi.org/10.1038/s41588-018-0048-5.
Dutta RK, et al. A somatic mutation in CLCN2 identified in a sporadic aldosterone-producing adenoma. Eur J Endocrinol. 2019;181(5):K37–41. https://doi.org/10.1530/EJE-19-0377.
Rege J, et al. Identification of Somatic Mutations in CLCN2 in Aldosterone-Producing Adenomas. J Endocr Soc. 2020;4(10):bvaa123. https://doi.org/10.1210/jendso/bvaa123.
Tadjine M, et al. Frequent mutations of beta-catenin gene in sporadic secreting adrenocortical adenomas. Clin Endocrinol (Oxf). 2008;68(2):264–70. https://doi.org/10.1111/j.1365-2265.2007.03033.x43.
Shimada H, et al. Molecular Mechanisms of Functional Adrenocortical Adenoma and Carcinoma: Genetic Characterization and Intracellular Signaling Pathway. Biomedicines. 2021;9(8). https://doi.org/10.3390/biomedicines9080892.
Rhayem Y, et al. PRKACA Somatic Mutations Are Rare Findings in Aldosterone-Producing Adenomas. J Clin Endocrinol Metab. 2016;101(8):3010–7. https://doi.org/10.1210/jc.2016-1700.
Nakajima Y, et al. GNAS mutations in adrenal aldosterone-producing adenomas. Endocr J. 2016;63(2):199–204. https://doi.org/10.1507/endocrj.EJ15-0642.
Zilbermint M, et al. Primary Aldosteronism and ARMC5 Variants. J Clin Endocrinol Metab. 2015;100(6):E900–9. https://doi.org/10.1210/jc.2014-416747.
Mulatero P, et al. ARMC5 mutation analysis in patients with primary aldosteronism and bilateral adrenal lesions. J Hum Hypertens. 2016;30(6):374–8. https://doi.org/10.1038/jhh.2015.9848.
Joseph JJ, et al. The Association of ARMC5 with the Renin-Angiotensin-Aldosterone System, Blood Pressure, and Glycemia in African Americans. J Clin Endocrinol Metab. 2020;105(8). https://doi.org/10.1210/clinem/dgaa290.
Hattangady NG, et al. Molecular and Electrophysiological Analyses of ATP2B4 Gene Variants in Bilateral Adrenal Hyperaldosteronism. Horm Cancer. 2020;11(1):52–62. https://doi.org/10.1007/s12672-019-00375-050.
•• Rassi-Cruz M, et al. Phosphodiesterase 2A and 3B variants are associated with primary aldosteronism. Endocr Relat Cancer. 2021;28(1):1–13. https://doi.org/10.1530/ERC-20-038451. This study provides novel genetic findings of the genetic etiology of primary hyperaldosteronism.
Hong AR, et al. Genetics of Aldosterone-Producing Adenoma in Korean Patients. PLoS ONE. 2016;11(1): e0147590. https://doi.org/10.1371/journal.pone.0147590.
Wu VC, et al. Prevalence and clinical correlates of somatic mutation in aldosterone producing adenoma-Taiwanese population. Sci Rep. 2015;5:11396. https://doi.org/10.1038/srep11396.
Nanba K, et al. Genetic Characteristics of Aldosterone-Producing Adenomas in Blacks. Hypertension. 2019;73(4):885–92. https://doi.org/10.1161/HYPERTENSIONAHA.118.12070.
Okamura T, et al. Characteristics of Japanese aldosterone-producing adenomas with KCNJ5 mutations. Endocr J. 2017;64(1):39–47. https://doi.org/10.1507/endocrj.EJ16-0243.
Warachit W, et al. Prevalence of Somatic KCNJ5 Mutations in Thai Patients With Aldosterone-Producing Adrenal Adenomas. J Endocr Soc. 2018;2(10):1137–46. https://doi.org/10.1210/js.2018-00097.
Lifton RP, et al. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature. 1992;355(6357):262–5. https://doi.org/10.1038/355262a0.
Sutherland DJ, Ruse JL, Laidlaw JC. Hypertension, increased aldosterone secretion and low plasma renin activity relieved by dexamethasone. Can Med Assoc J. 1966;95(22):1109–19.
Stowasser M, et al. Evidence for abnormal left ventricular structure and function in normotensive individuals with familial hyperaldosteronism type I. J Clin Endocrinol Metab. 2005;90(9):5070–6. https://doi.org/10.1210/jc.2005-0681.
Stowasser M, et al. Familial hyperaldosteronism type II: five families with a new variety of primary aldosteronism. Clin Exp Pharmacol Physiol. 1992;19(5):319–22. https://doi.org/10.1111/j.1440-1681.1992.tb00462.x.
Geller DS, et al. A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab. 2008;93(8):3117–23. https://doi.org/10.1210/jc.2008-0594.
Mulatero P, et al. KCNJ5 mutations in European families with nonglucocorticoid remediable familial hyperaldosteronism. Hypertension. 2012;59(2):235–40. https://doi.org/10.1161/HYPERTENSIONAHA.111.183996.
Monticone S, et al. A Novel Y152C KCNJ5 mutation responsible for familial hyperaldosteronism type III. J Clin Endocrinol Metab. 2013;98(11):E1861–5. https://doi.org/10.1210/jc.2013-2428.
Scholl UI, et al. Hypertension with or without adrenal hyperplasia due to different inherited mutations in the potassium channel KCNJ5. Proc Natl Acad Sci U S A. 2012;109(7):2533–8. https://doi.org/10.1073/pnas.1121407109.
Charmandari E, et al. A novel point mutation in the KCNJ5 gene causing primary hyperaldosteronism and early-onset autosomal dominant hypertension. J Clin Endocrinol Metab. 2012;97(8):E1532–9. https://doi.org/10.1210/jc.2012-1334.
Adachi M, et al. Discordant genotype-phenotype correlation in familial hyperaldosteronism type III with KCNJ5 gene mutation: a patient report and review of the literature. Horm Res Paediatr. 2014;82(2):138–42. https://doi.org/10.1159/000358197.
Sertedaki A, et al. Functional characterization of two novel germline mutations of the KCNJ5 gene in hypertensive patients without primary aldosteronism but with ACTH-dependent aldosterone hypersecretion. Clin Endocrinol (Oxf). 2016;85(6):845–51. https://doi.org/10.1111/cen.13132.
Maria AG, et al. Mosaicism for KCNJ5 Causing Early-Onset Primary Aldosteronism due to Bilateral Adrenocortical Hyperplasia. Am J Hypertens. 2020;33(2):124–30. https://doi.org/10.1093/ajh/hpz172.
Acknowledgements
The authors would like to thank Yolanda L. Jones, National Institutes of Health Library, for editing assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
Dr. Hannah-Shmouni is a member of the Endocrine Hypertension Subcommittee of the Canadian Hypertension Guidelines (Hypertension Canada) and has no relevant disclosures to report. Dr. Stratakis holds patents on the PRKAR1A, PDE11A, and GPR101 genes and/or their function and has received research funding from Pfizer Inc. on the genetics and treatment of abnormalities of growth hormone secretion. Dr. Stratakis is currently employed by ELPEN Pharmaceuticals and has received consulting fees from Sync, Lundbeck, and Sandoz. Dr. Stratakis also reports grant support from the NIH. Dr. Faucz holds a patent on the GPR101 gene and its function. Dr. Pitsava has no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any new human or animal trial study results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hypertension
Rights and permissions
About this article
Cite this article
Pitsava, G., Faucz, F.R., Stratakis, C.A. et al. Update on the Genetics of Primary Aldosteronism and Aldosterone-Producing Adenomas. Curr Cardiol Rep 24, 1189–1195 (2022). https://doi.org/10.1007/s11886-022-01735-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-022-01735-z