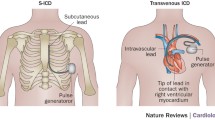
Abstract
Purpose of Review
While the subcutaneous (S-) implantable cardioverter-defibrillator (ICDs) is an alternative to the transvenous (TV-) ICD in many patients, optimal use remains unclear. In this review, we summarize recent clinically relevant data on sensing algorithms, inappropriate shocks, defibrillation testing, and battery and electrode failures.
Recent Findings
Changes in sensing algorithms and S-ICD programming have significantly decreased inappropriate shock rates. Avoiding fat below the S-ICD coil and can is key for reducing the defibrillation threshold. While S-ICD battery and electrode failures have resulted in recalls, system components remain commercially available since failure rates are low and no other similar devices are available.
Summary
The S-ICD is a good alternative to the TV-ICD for many patients, and particularly in light of recently developed device algorithms and improvements in implant technique. Future research will need to better understand: the impact of S-ICD electrode and battery failures and the potential for integrating leadless pacing into a modular S-ICD platform.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Friedman DJ, Parzynski CS, Varosy PD, Prutkin JM, Patton KK, Mithani A, et al. Trends and In-Hospital Outcomes Associated With Adoption of the Subcutaneous Implantable Cardioverter Defibrillator in the United States. JAMA Cardiol. 2016;1(8):900–11.
Burke MC, Gold MR, Knight BP, Barr CS, Theuns DA, Boersma LV, et al. Safety and Efficacy of the Totally Subcutaneous Implantable Defibrillator: 2-Year Results From a Pooled Analysis of the IDE Study and EFFORTLESS Registry. J Am Coll Cardiol. 2015;65(16):1605–15.
Lambiase PD, Barr C, Theuns DA, Knops R, Neuzil P, Johansen JB, et al. Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the EFFORTLESS S-ICD Registry. Eur Heart J. 2014;35(25):1657–65.
•• Knops RE, Olde Nordkamp LRA, Delnoy PHM, Boersma LVA, Kuschyk J, El-Chami MF, et al. Subcutaneous or Transvenous Defibrillator Therapy. N Engl J Med. 2020;383(6):526–36. The first and only published randomized trial of S-ICD and TV-ICD; this study demonstrated the primary outcome of device related complication or inappropriate shocks was similar in both groups.
• Friedman DJ QL, Parzynski C, Heist EK, Russo AM, Ranasinghe I., Zeitler EP, Minges KE, Akar JG, Freeman JV, Curtis JP, Al-Khatib SM. Longitudinal Outcomes of Subcutaneous or Transvenous Implantable Cardioverter-Defibrillators in Older Patients. Journal of the American College of Cardiology. 2022;79(11):1050–9. This observational study demonstrated rehospitalization, device re-operation for any reason, and death, were similar among older patients who receieved a S-ICD or TV-ICD.
Brisben AJ, Burke MC, Knight BP, Hahn SJ, Herrmann KL, Allavatam V, et al. A new algorithm to reduce inappropriate therapy in the S-ICD system. J Cardiovasc Electrophysiol. 2015;26(4):417–23.
Gold MR, Weiss R, Theuns DA, Smith W, Leon A, Knight BP, et al. Use of a discrimination algorithm to reduce inappropriate shocks with a subcutaneous implantable cardioverter-defibrillator. Heart rhythm : the official journal of the Heart Rhythm Society. 2014;11(8):1352–8.
Theuns D, Brouwer TF, Jones PW, Allavatam V, Donnelley S, Auricchio A, et al. Prospective blinded evaluation of a novel sensing methodology designed to reduce inappropriate shocks by the subcutaneous implantable cardioverter-defibrillator. Heart rhythm : the official journal of the Heart Rhythm Society. 2018;15(10):1515–22.
•• Gold MR, Lambiase PD, El-Chami MF, Knops RE, Aasbo JD, Bongiorni MG, et al. Primary Results From the Understanding Outcomes With the S-ICD in Primary Prevention Patients With Low Ejection Fraction (UNTOUCHED) Trial. Circulation. 2021;143(1):7–17. This prospective, single arm study demonstrated that with contemporary programming and sensing algorithms, the inappropriate shock rate with the S-ICD was similar to TV-ICD, based on a pre-specified performance goal.
Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, et al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367(24):2275–83.
Baalman SWE, Mittal S, Boersma LVA, Perschbacher D, Brisben AJ, Mahajan D, et al. Real-world performance of the atrial fibrillation monitor in patients with a subcutaneous ICD. Pacing Clin Electrophysiol. 2020;43(12):1467–75.
Rordorf R, Casula M, Pezza L, Fortuni F, Sanzo A, Savastano S, et al. Subcutaneous versus transvenous implantable defibrillator: An updated meta-analysis. Heart rhythm : the official journal of the Heart Rhythm Society. 2021;18(3):382–91.
Zeitler EP, Friedman DJ, Loring Z, Campbell KB, Goldstein SA, Wegermann ZK, et al. Complications involving the subcutaneous implantable cardioverter-defibrillator: Lessons learned from MAUDE. Heart rhythm : the official journal of the Heart Rhythm Society. 2020;17(3):447–54.
Boersma L, Burke MC, Neuzil P, Lambiase P, Friehling T, Theuns DA, et al. Infection and mortality after implantation of a subcutaneous ICD after transvenous ICD extraction. Heart Rhythm. 2016;13(1):157–64.
Pun PH, Parzynski CS, Friedman DJ, Sanders G, Curtis JP, Al-Khatib SM. Trends in Use and In-Hospital Outcomes of Subcutaneous Implantable Cardioverter Defibrillators in Patients Undergoing Long-Term Dialysis. Clin J Am Soc Nephrol. 2020;15(11):1622–30.
Migliore F, Allocca G, Calzolari V, Crosato M, Facchin D, Daleffe E, et al. Intermuscular Two-Incision Technique for Subcutaneous Implantable Cardioverter Defibrillator Implantation: Results from a Multicenter Registry. Pacing Clin Electrophysiol. 2017;40(3):278–85.
Bardy GH, Smith WM, Hood MA, Crozier IG, Melton IC, Jordaens L, et al. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med. 2010;363(1):36–44.
Weiss R, Knight BP, Gold MR, Leon AR, Herre JM, Hood M, et al. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation. 2013;128(9):944–53.
Wilkoff BL, Fauchier L, Stiles MK, Morillo CA, Al-Khatib SM, Almendral J, et al. 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Heart rhythm : the official journal of the Heart Rhythm Society. 2016;13(2):e50-86.
Friedman DJ, Parzynski CS, Heist EK, Russo AM, Akar JG, Freeman JV, et al. Ventricular Fibrillation Conversion Testing After Implantation of a Subcutaneous Implantable Cardioverter Defibrillator: Report From the National Cardiovascular Data Registry. Circulation. 2018;137(23):2463–77.
Gold MR, Aasbo JD, El-Chami MF, Niebauer M, Herre J, Prutkin JM, et al. Subcutaneous implantable cardioverter-defibrillator Post-Approval Study: Clinical characteristics and perioperative results. Heart rhythm : the official journal of the Heart Rhythm Society. 2017.
Hsu JC, Marcus GM, Al-Khatib SM, Wang Y, Curtis JP, Sood N, et al. Predictors of an inadequate defibrillation safety margin at ICD implantation: insights from the National Cardiovascular Data Registry. J Am Coll Cardiol. 2014;64(3):256–64.
Heist EK, Belalcazar A, Stahl W, Brouwer TF, Knops RE. Determinants of Subcutaneous Implantable Cardioverter-Defibrillator Efficacy: A Computer Modeling Study. J Am Coll Cardiol EP. 2017;3:405–14.
Quast ABE, Baalman SWE, Brouwer TF, Smeding L, Wilde AAM, Burke MC, et al. A novel tool to evaluate the implant position and predict defibrillation success of the subcutaneous implantable cardioverter-defibrillator: The PRAETORIAN score. Heart rhythm : the official journal of the Heart Rhythm Society. 2019;16(3):403–10.
van der Stuijt W, Quast ABE, Baalman SWE, de Wilde KC, Brouwer TF, Wilde AAM, et al. Complications related to elective generator replacement of the subcutaneous implantable defibrillator. Europace. 2021;23(3):395–9.
Rudic B, Tulumen E, Fastenrath F, Akin I, Borggrefe M, Kuschyk J. Defibrillation failure in patients undergoing replacement of subcutaneous defibrillator pulse generator. Heart rhythm : the official journal of the Heart Rhythm Society. 2020;17(3):455–9.
Maisel WH, Sweeney MO, Stevenson WG, Ellison KE, Epstein LM. Recalls and safety alerts involving pacemakers and implantable cardioverter-defibrillator generators. JAMA. 2001;286(7):793–9.
Maisel WH, Stevenson WG, Epstein LM. Changing trends in pacemaker and implantable cardioverter defibrillator generator advisories. Pacing Clin Electrophysiol. 2002;25(12):1670–8.
Zeitler EP, Patel D, Hasselblad V, Sanders GD, Al-Khatib SM. Complications from prophylactic replacement of cardiac implantable electronic device generators in response to United States Food and Drug Administration recall: A systematic review and meta-analysis. Heart Rhythm. 2015;12(7):1558–64.
Zeitler EP, Pokorney SD, Zhou K, Lewis RK, Greenfield RA, Daubert JP, et al. Cable externalization and electrical failure of the Riata family of implantable cardioverter-defibrillator leads: A systematic review and meta-analysis. Heart Rhythm. 2015;12(6):1233–40.
Kay GN, Brinker JA, Kawanishi DT, Love CJ, Lloyd MA, Reeves RC, et al. Risks of spontaneous injury and extraction of an active fixation pacemaker lead: report of the Accufix Multicenter Clinical Study and Worldwide Registry. Circulation. 1999;100(23):2344–52.
Providencia R, Kramer DB, Pimenta D, Babu GG, Hatfield LA, Ioannou A, et al. Transvenous Implantable Cardioverter-Defibrillator (ICD) Lead Performance: A Meta-Analysis of Observational Studies. J Am Heart Assoc. 2015;4(11).
U.S. Food & Drug Administration. Summary of Safety and Effectiveness Data (SSED): Subcutaneous Implantable Defibrillator. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf11/P110042B.pdf.
Burke MC, Aasbo JD, El-Chami MF, Weiss R, Dinerman J, Hanon S, et al. 1-Year Prospective Evaluation of Clinical Outcomes and Shocks: The Subcutaneous ICD Post Approval Study. JACC Clin Electrophysiol. 2020;6(12):1537–50.
Lambiase PD, Theuns DA, Murgatroyd F, Barr C, Eckardt L, Neuzil P, et al. Subcutaneous implantable cardioverter-defibrillators: long-term results of the EFFORTLESS study. European Heart Journal. 2022.
Boston Scientific. Rhythm Management Product Performance Report: 2021 Q4 Edition. 2021 October 18, 2021.
U.S. Food & Drug Administration. Boston Scientific recalls EMBLEM S-ICD subcutaneous electrode (model 3501) due to risk of fractures 2020. Available from: https://www.fda.gov/medical-devices/medical-device-recalls/boston-scientific-recalls-emblem-s-icd-subcutaneous-electrode-model-3501-due-risk-fractures.
Kella DK, Stambler BS. Subcutaneous implantable cardioverter-defibrillator electrode fracture: Follow-up, troubleshooting, and evaluation. J Cardiovasc Electrophysiol. 2021;32(5):1452–7.
U.S. Food & Drug Administration. Boston Scientific Corporation recalls EMBLEM S-ICD (subcutaneous implantable cardioverter defibrillator) system due to risk of short-circuit 2020. Available from: https://www.fda.gov/medical-devices/medical-device-recalls/boston-scientific-corporation-recalls-emblem-s-icd-subcutaneous-implantable-cardioverter.
Ip JE. Premature battery depletion of EMBLEM subcutaneous implantable cardioverter-defibrillators. J Cardiovasc Electrophysiol. 2021;32(3):565–7.
Gasperetti A, Schiavone M, Ziacchi M, Vogler J, Breitenstein A, Laredo M, et al. Long-term complications in patients implanted with subcutaneous implantable cardioverter-defibrillators: Real-world data from the extended ELISIR experience. Heart Rhythm. 2021;18(12):2050–8.
Boston Scientific. Important Medical Device Advisory: EMBLEM S-ICD Subcutaneous Electrodes (Model 3501). Available from: https://www.bostonscientific.com/content/dam/bostonscientific/quality/dlt/reg-code-228/2020Dec_BSC_EmblemElectrode3501_PhysLtr_Final.pdf.
Weberndorfer V, Nyffenegger T, Russi I, Brinkert M, Berte B, Toggweiler S, et al. First time description of early lead failure of the Linox Smart lead compared to other contemporary high-voltage leads. J Interv Card Electrophysiol. 2018;52(2):173–7.
Boston Scientific. Important Medical Device Advisory: EMBLEM S-ICD Models A209 and A219 2020. Available from: https://www.bostonscientific.com/content/dam/bostonscientific/quality/dlt/reg-code-228/2020Dec_BSC_EmblemPBD_PhysLtr_US_Final.pdf.
Jensen T, Chin J, Ashby L, Dolan D. Decision Memo for Implantable Automatic Defibrillators (CAG-00157R4). Centers for Medicare and Medicaid Services; 2018.
Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72(14):1677–749.
Sears SF Jr, Conti JB. Quality of life and psychological functioning of icd patients. Heart. 2002;87(5):488–93.
Pedersen SS, Lambiase P, Boersma LV, Murgatroyd F, Johansen JB, Reeve H, et al. Evaluation oF FactORs ImpacTing CLinical Outcome and Cost EffectiveneSS of the S-ICD: design and rationale of the EFFORTLESS S-ICD Registry. Pacing Clin Electrophysiol. 2012;35(5):574–9.
Pedersen SS, Mastenbroek MH, Carter N, Barr C, Neuzil P, Scholten M, et al. A Comparison of the Quality of Life of Patients With an Entirely Subcutaneous Implantable Defibrillator System Versus a Transvenous System (from the EFFORTLESS S-ICD Quality of Life Substudy). Am J Cardiol. 2016;118(4):520–6.
Srinivasan NT, Patel KH, Qamar K, Taylor A, Baca M, Providencia R, et al. Disease Severity and Exercise Testing Reduce Subcutaneous Implantable Cardioverter-Defibrillator Left Sternal ECG Screening Success in Hypertrophic Cardiomyopathy. Circ Arrhythm Electrophysiol. 2017;10(4).
Lambiase PD, Gold MR, Hood M, Boersma L, Theuns D, Burke MC, et al. Evaluation of subcutaneous ICD early performance in hypertrophic cardiomyopathy from the pooled EFFORTLESS and IDE cohorts. Heart rhythm : the official journal of the Heart Rhythm Society. 2016;13(5):1066–74.
Nazer B, Dale Z, Carrassa G, Reza N, Ustunkaya T, Papoutsidakis N, et al. Appropriate and inappropriate shocks in hypertrophic cardiomyopathy patients with subcutaneous implantable cardioverter-defibrillators: An international multicenter study. Heart rhythm : the official journal of the Heart Rhythm Society. 2020;17(7):1107–14.
Rudic B, Tulumen E, Berlin V, Roger S, Stach K, Liebe V, et al. Low Prevalence of Inappropriate Shocks in Patients With Inherited Arrhythmia Syndromes With the Subcutaneous Implantable Defibrillator Single Center Experience and Long-Term Follow-Up. J Am Heart Assoc. 2017;6(10).
Weinstock J, Bader YH, Maron MS, Rowin EJ, Link MS. Subcutaneous Implantable Cardioverter Defibrillator in Patients With Hypertrophic Cardiomyopathy: An Initial Experience. J Am Heart Assoc. 2016;5(2).
Jankelson L, Garber L, Sherrid M, Massera D, Jones P, Barbhaiya C, et al. Subcutaneous versus transvenous implantable defibrillator in patients with hypertrophic cardiomyopathy. Heart rhythm : the official journal of the Heart Rhythm Society. 2022.
Lambiase PD, Eckardt L, Theuns DA, Betts TR, Kyriacou AL, Duffy E, et al. Evaluation of subcutaneous implantable cardioverter-defibrillator performance in patients with ion channelopathies from the EFFORTLESS cohort and comparison with a meta-analysis of transvenous ICD outcomes. Heart Rhythm O2. 2020;1(5):326–35.
Conte G, Kawabata M, de Asmundis C, Taravelli E, Petracca F, Ruggiero D, et al. High rate of subcutaneous implantable cardioverter-defibrillator sensing screening failure in patients with Brugada syndrome: a comparison with other inherited primary arrhythmia syndromes. Europace. 2018;20(7):1188–93.
Conte G, Cattaneo F, de Asmundis C, Berne P, Vicentini A, Namdar M, et al. Impact of SMART Pass filter in patients with ajmaline-induced Brugada syndrome and subcutaneous implantable cardioverter-defibrillator eligibility failure: results from a prospective multicentre study. Europace. 2021.
El-Chami MF, Burke MC, Herre JM, Shah MH, Sadhu A, Niebauer MJ, et al. Outcomes of subcutaneous implantable cardioverter-defibrillator in dialysis patients: Results from the S-ICD post-approval study. Heart rhythm : the official journal of the Heart Rhythm Society. 2020;17(9):1566–74.
Black-Maier E, Lewis RK, Barnett AS, Pokorney SD, Sun AY, Koontz JI, et al. Subcutaneous implantable cardioverter-defibrillator troubleshooting in patients with a left ventricular assist device: A case series and systematic review. Heart rhythm : the official journal of the Heart Rhythm Society. 2020;17(9):1536–44.
von Alvensleben JC, Dechert B, Bradley DJ, Fish FA, Moore JP, Pilcher TA, et al. Subcutaneous Implantable Cardioverter-Defibrillators in Pediatrics and Congenital Heart Disease: A Pediatric and Congenital Electrophysiology Society Multicenter Review. JACC Clin Electrophysiol. 2020;6(14):1752–61.
Alvensleben JCv, Dechert B, Bradley DJ, Fish FA, Moore JP, Pilcher TA, et al. Subcutaneous Implantable Cardioverter-Defibrillators in Pediatrics and Congenital Heart Disease. JACC: Clinical Electrophysiology. 2020;6(14):1752–61.
Boston Scientific C. Effectiveness of the EMPOWER™ Modular Pacing System and EMBLEM™ Subcutaneous ICD to Communicate Antitachycardia Pacing 2023. [Updated January 31]. Available from: https://ClinicalTrials.gov/show/NCT04798768.
Boersma L, Barr C, Knops R, Theuns D, Eckardt L, Neuzil P, et al. Implant and Midterm Outcomes of the Subcutaneous Implantable Cardioverter-Defibrillator Registry: The EFFORTLESS Study. J Am Coll Cardiol. 2017;70(7):830–41.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Friedman has received: research grants from the American Heart Association, National Cardiovascular Data Registry, Johnson & Johnson, Boston Scientific, Abbott, Medtronic, Merit Medical, and Biosense Webster; and consulting fees from Abbott, AtriCure, Sanofi, and NI Medical. Dr. Zeitler has received consulting fees from Medtronic, Inc., Biosense Webster, and Arena Pharmaceuticals (now Pfizer); support for travel to educational meetings from Medtronic Inc., Abbott, and Heart Rhythm Society; research grants from Boston Scientific and the National Cardiovascular Data Registry; and nonfinancial research support from Biosense Webster and Sanofi. Dr. Tully reports no conflicts.
Human and Animal Rights and Informed Consent
Some of the papers in this review were published by the authors. However, these were retrospective studies that received appropriate IRB approval and no informed consent was required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Invasive Electrophysiology and Pacing
Rights and permissions
About this article
Cite this article
Friedman, D.J., Tully, A.S. & Zeitler, E.P. Subcutaneous and Transvenous ICDs: an Update on Contemporary Questions and Controversies. Curr Cardiol Rep 24, 947–958 (2022). https://doi.org/10.1007/s11886-022-01712-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-022-01712-6