Abstract
Purpose of Review
Since 2015, when ESC guidelines for the diagnosis and management of pericardial diseases were published, ongoing research has enhanced the current state of knowledge on acute pericarditis. This review is an update on the latest developments in this field.
Recent Findings
In recurrent acute pericarditis, autoinflammation has been included among causative mechanisms restricting the vague diagnoses of “idiopathic” pericarditis. Cardiac magnetic resonance that detects ongoing pericardial inflammation may guide treatment in difficult-to-treat patients. Development of risk scores may assist identification of patients at high risk for complicated pericarditis, who should be closely monitored and aggressively treated. Treatment with IL-1 inhibitors has been proven efficacious in recurrent forms with a good safety profile. Finally, acute pericarditis has recently attracted great interest as it has been reported among side effects post COVID-19 vaccination and may also complicate SARS-CoV-2 infection.
Summary
Recent advancements in acute pericarditis have contributed to a better understanding of the disease allowing a tailored to the individual patient approach. However, there are still unsolved questions that require further research.
Similar content being viewed by others
Introduction
Acute pericarditis is the most common inflammatory heart disorder, ahead of acute myocarditis and infective endocarditis [1]. In the Western world, the incidence of acute pericarditis in the general population is estimated at approximately 27.7 cases per 100,000 subjects per year, whereas the standardized incidence rate of hospital admissions for pericarditis is 3.32 cases per 100,000 person-years [1,2,3]. Pericarditis, similarly to myocarditis, is more common among younger males. Sex hormones, mainly testosterone, appear to play a role in the different susceptibility of pericarditis in men and women [1, 4].
Acute pericarditis is an overall benign and self-limiting disease [1]. However, in some unfortunate instances, complications may occur either in the short term (such as cardiac tamponade which is a dreadful and potentially life-threatening complication if not promptly recognized and treated), mid-term (recurrent pericarditis), or long term (permanent constrictive pericarditis) [5]. The incidence of complications depends largely on the underlying etiology (higher in secondary forms), certain patient characteristics (age and sex), and treatment choice (such as use of glucocorticoids) [1, 4, 6•, 7]. Acute pericarditis may appear either as an isolated disease or in the context of a systemic disorder. In the latter circumstance, a multidisciplinary approach is of paramount importance for a favorable outcome. Indeed, in the so-called idiopathic (presumably viral) forms, cardiologists are involved in patient management, whereas in the secondary forms, a collaboration between cardiologists and other medical specialties is required (e.g., rheumatologists, oncologists) [8]. In contrast to first presenting acute pericarditis, recurrent pericarditis is a problematic and often difficult to treat condition [9]. After a first episode of acute pericarditis, the rate of first recurrence is reported at 15–30% of cases [10]. Notably, among patients with a first recurrence, a second is observed in 25–50%, and a further (third) in 20–40% [10]. In some unfortunate cases (approximately 5–10%), additional recurrences may appear with detrimental impact on patients’ quality of life, due to frequent emergency department or hospital admissions as well as complications related to medical treatment [10, 11]. Thus, the so-called glucocorticoid-dependent, colchicine-resistant recurrent pericarditis is a major challenge for contemporary cardiology and a field of intense ongoing research regarding pathophysiology, prompt recognition of these patients, and optimal treatment.
Etiology
The etiology of pericarditis includes infectious and non-infectious forms (Table 1) [1, 7]. Among infectious forms, the most common is viral pericarditis which accounts for approximately 80–85% of all pericarditis cases [1, 7]. A recent clinically apparent viral infection (upper respiratory tract infection or gastroenteritis) has been documented in approximately 40% of patients presenting with acute pericardial inflammation [12]. At the time of pericarditis diagnosis, however, viral infection cannot be confirmed with serology antibody testing since IgM antibodies are often no longer detectable [1]. Molecular techniques (such as polymerase chain reaction) on pericardial fluid or tissue after pericardiocentesis and pericardial biopsy respectively would be possibly able to identify the infectious agent, but this is an invasive and non-recommended approach for an overall benign disorder [1].
According to the current guidelines, the routine identification of the causative viral agent, with the possible exception of hepatitis C virus and HIV, is not recommended, since it does not affect treatment decisions and prognosis [1]. Taking into account the above clues, in the contemporary literature, the terms viral and idiopathic pericarditis are considered synonyms, assuming that idiopathic is of a presumably (but not proven) viral etiology [13]. It is stressed that in the etiology search of acute pericarditis, the local epidemiological data should always be considered. For instance, in the developing countries, the most common form of pericarditis is tuberculous, which accounts for approximately 70–80% of cases with the rate increasing to 90% in HIV-positive patients [7].
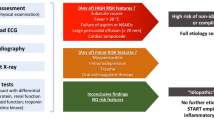
As already mentioned, the identification of the underlying etiology is of paramount importance for a tailored treatment plan. In this line, the identification of patients who probably suffer from pericarditis in the context of a systemic disorder is strongly recommended. Features suggesting a secondary (specific) etiology are summarized in Table 2 [1]. Patients presenting with at least one of those findings should be hospitalized, monitored for complications (mainly cardiac tamponade), and subjected to an extensive clinically oriented diagnostic work-up, including blood work and second-level imaging in order to reveal potential specific etiologies [1]. Among all comers with acute pericarditis, it is estimated that at least 1 high-risk criterion is found in approximately 15% of total cases [14].
Besides the well-recognized etiologies of acute pericarditis, two important new etiologies have been described in the last 2 years. The first is infectious, namely SARS-CoV-2, and the second non-infectious, which is the vaccine against COVID-19 and especially the mRNA vaccine platforms [15, 16•]. With these 2 novel etiologies, pericarditis along with myocarditis have taken front stage. In the setting of COVID-19 infection, real-world data showed that new-onset pericarditis developed in 1.5% of cases. The 6-month all-cause mortality was 15.5% for patients with pericarditis vs. 6.7% in matched controls with COVID-19 but without pericarditis [17]. In another large study, the incidence rate ratio of pericarditis 1–28 days following a SARS-CoV-2 positive test was 2.79 [18••]. Concerning vaccination against COVID-19 (any vaccine), in the latter study which included 38,615,491 adults that had been vaccinated with at least one dose, 0.001% were hospitalized with pericarditis in the 1–28 days after any dose of vaccine [18••]. Similar findings were recorded in another study including 2,000,287 individuals receiving at least 1 vaccination dose [19]. Pericarditis developed in 37 cases, most often (~ 60%) after the second immunization with a median onset of 20 days (IQR, 6.0–41.0 days) after the most recent vaccination. Notably, in contrast to acute myocarditis, pericarditis was more common in older subjects [19]. The above data provide indisputable evidence that benefits of COVID-19 vaccination counterbalance any concerns about the possible but definitely rare vaccine-induced pericarditis and inflammatory heart disease overall.
In our institutional experience stemming from a referral center for pericardial diseases in a large urban area, the overall clinical course in subjects presenting with pericarditis following COVID-19 vaccination does not differ compared to common viral pericarditis [20].
Diagnosis
In the absence of a specific biomarker, such as troponins for myocardial necrosis, the diagnosis of acute pericarditis (either first episode or recurrences) is based on clinical and imaging findings. According to the 2015 ESC guidelines for the diagnosis and management of pericardial diseases, the diagnosis is established by the presence of at least 2 out of the 4 criteria, which are depicted in Table 2 and Supplemental Fig. 1 [1]. Among them, the most common is pleuritic chest pain which typically worsens with inspiration and is relieved in the sitting position and leaning forward. It is observed in more than approximately 90–95% of cases [1, 7, 21].
The second is auscultation of pericardial rubs, which although pathognomonic for acute pericarditis, are encountered in approximately 30% of patients. However, it is an intermittent finding that requires expertise for its detection [1, 22]. Pericardial effusion is found in 60–80% of cases and is taken into consideration if first appearing or worsening during the course of the disease [1, 14, 23]. In most cases (~ 80%), the effusion is small-sized, less often moderate (10%) or large (10%) with or without evidence of cardiac tamponade [14]. It should be emphasized that the size of the effusion is calculated semi-quantitatively with transthoracic echocardiography by measuring the largest diameter of the effusion in end-diastole [1]. The effusion appears as an echo-free space between the visceral and parietal pericardial layers and is considered small if the space in question is less than 1 cm, moderate if between 1 and 2 cm, and large if greater than 2 cm [1]. Quantitative assessment of pericardial fluid is obtained with computed tomography with a specific software, and CMR [1].
Electrocardiography is a valuable tool for the diagnosis of acute pericarditis and a differential diagnosis of alternative etiologies of chest pain. Classically, 4 phases of approximately 1 week each are described. However, the duration of each phase varies largely between patients and depends on the time of starting medical treatment, its effectiveness, and eventual appearance of complications [1, 21]. Typical findings include diffuse concave ST segment elevation without coronary artery territorial distribution, PR segment depression (which is the most specific finding in acute pericarditis), no reciprocal ST segment depression, ST segment depression in lead aVR and occasionally in V1 lead, and notably, lack of Q wave development. In the second phase, the ST and PR segments become isoelectric, in the third T wave, inversion is observed, and in the last phase, the electrocardiogram normalizes (the latter phase in some instances may appear later on). Unfortunately, this typical series of events is observed in 50–60% of cases and in several patients, the electrocardiogram depicts non-specific findings [1, 21, 22].
Apart from the aforementioned criteria, additional less specific symptoms may be encountered in the individual patient such as fever, cough, and dyspnea along with other findings suggesting eventually a secondary form such as arthritis, rush, and lymph node enlargement. According to the ESC guidelines, inflammatory markers and evidence of myocardial inflammation by an imaging modality (CT, CMR) are considered supportive findings and at present are not included among the main diagnostic criteria [1]. For CMR, the main reason for exclusion is the limited availability and cost. Regarding C-reactive protein, apart from its low specificity (as it elevates in inflammatory conditions of any etiology), another concern is that in approximately 22% of cases, it is found within normal limits at the time of presentation [24]. This is due to CRP synthesis rate kinetics, as CRP levels start rising beyond normal values approximately 6 h after inflammation onset. However, CRP values have been found to be elevated in 96% of patients with acute pericarditis if measured at least 12 h after symptom onset [25].
Finally in assessing a patient with suspected acute pericarditis, age and sex peculiarities should be considered. For instance, it has been shown that elderly females with acute pericarditis may present with dyspnea rather than chest pain, without rubs and fever and with non-specific findings in the electrocardiogram, rendering the diagnosis of pericarditis challenging with the adoption of the established criteria [4].
Diagnostic Work-up
In a patient with a suspected first episode of acute pericarditis, routine (or first-level investigations) diagnostic work-up according to the ESC guidelines should include detailed medical history, physical examination with emphasis on heart auscultation, chest X-ray, electrocardiogram, echocardiography, and routine blood tests which should include markers of inflammation (mainly CRP), troponin, and thyroid hormones [1]. In the presence of one or more high-risk criteria, a more extensive work-up should be considered due to the higher prevalence of a secondary condition in these patients [1]. The same holds true for patients with recurrent acute pericarditis where secondary conditions are more prevalent as well [5]. When a secondary condition is suspected, then a broader blood work should be performed including serologic markers for autoimmune diseases, thyroid hormones, QuantiFERON-TB Gold, and tumor markers. CCT or cardiac MRI are also included among the second-level investigations and should be adopted when routine (first level) testing is not sufficient for diagnostic purposes [1].
CCT offers the advantage of superior spatial resolution. It is the most accurate imaging technique for the measurement of pericardial thickness and the evaluation of pericardial calcifications (in terms of presence and burden) which is particularly helpful in planning surgical pericardiectomy. In contrast, it is not indicated in the evaluation of inflammatory pericardial conditions [26]. Cardiac MRI with its ability of tissue characterization, apart from diagnostic clues, offers invaluable information concerning treatment guidance [10, 26, 27••]. Actually, depending on the presence of pericardial edema in the T2 sequences and late gadolinium enhancement, the stage of disease and the effectiveness of treatment are evaluated, especially in cases of recurrent disease. In specific, during the acute phase of pericarditis, both edema and LGE are observed. Resolution of edema with LGE persistence dictates a subacute/chronic phase of the disease, whereas the absence of both edema and LGE dictates healing [1, 10, 26, 27••]. Cardiac MRI is also a valuable tool in the assessment of effusive-constrictive pericarditis, an uncommon disorder identified by pericardial effusion causing tamponade combined with thickened visceral pericardium causing constriction, which is typically unveiled upon pericardial drainage. The presence of pericardial edema and LDH as depicted by MRI suggest a possible reversibility of the condition with anti-inflammatory treatment [1, 26].
In an individualized fashion, tumor markers, PET-CT, and pericardial biopsy may be performed [1]. It should be mentioned that “idiopathic” pericarditis is not a life-long diagnosis and difficult-to-treat patients with multiple recurrences should be periodically reassessed to exclude the development of a secondary condition. In this subgroup of patients, it has been shown that in approximately 10% of cases, an occult secondary disorder may emerge, which most often is an autoimmune disorder (such as Sjögren syndrome and rheumatoid arthritis in a relevant case series) [28].
Differential Diagnosis
The differential diagnosis encompasses practically all cases of acute chest pain, including myocardial ischemia/infarction, pulmonary embolism, aortic dissection, inflammatory conditions of the chest (pneumonia), and benign conditions such as gastroesophageal reflux and musculoskeletal pain [1, 21, 22]. In most cases, based on the first-level investigations for pericarditis i.e., electrocardiography, chest X-ray, echocardiography, and routine blood work plus CRP and troponin, it is possible to obtain a correct diagnosis [1]. Nevertheless, in the individual patient, additional tests may be needed such as D-dimer measurement and chest CT among others [1]. It is however emphasized that in certain clinical scenarios, the exclusion of myocardial infarction is quite challenging. In particular, although the diagnosis of acute pericarditis is rather straightforward in a young male without risk factors for coronary artery disease presenting with chest pain and ST segment elevation, this does not hold always true for a middle aged or elderly man with a history (or multiple risk factors) of coronary artery disease presenting with a similar electrocardiogram. In this specific scenario, the exclusion of coronary artery obstruction with coronary arteriography is sometimes mandatory. In a study, coronary arteriography was performed in ~ 17% of patients which were finally diagnosed with acute pericarditis [29]. The relevant rate in those presenting with ST segment elevation was ~ 25%. This rate dictates the diagnostic challenges in suspected acute pericarditis in the everyday clinical practice [29].
Patients with a clinical picture of acute pericarditis with elevated troponin levels but without impairment of left ventricular ejection fraction nor left ventricular wall motion abnormalities are being diagnosed with myopericarditis [1, 7]. On the other hand, patients with pleuritic chest pain, with or without pericardial effusion, but with evidence of left or biventricular function abnormalities, are diagnosed with perimyocarditis and are managed in a similar way to pure myocarditis patients [7]. The subgroup of myopericarditis patients is treated in the same way as “pure” acute pericarditis. However, according to the ESC guidelines, the lower effective dose of NSAIDs for the shorter possible period should be administered since it has been shown in animal models that NSAIDs may enhance inflammation and increase mortality [1]. Moreover, the currently available data to recommend the use of colchicine in this setting are insufficient [1]. From a clinical perspective, the latter patients do not seem to have a worse prognosis compared to the acute pericarditis counterpart and interestingly, they depict less often recurrences (11% vs. 32%) after a first episode [30]. However, performing cardiac CMR in myopericarditis patients is reasonable, in order to assess the presence and extent of myocardial involvement [1].
Treatment
The treatment principles of acute pericarditis are depicted in Fig. 1. Exercise restriction is recommended in the acute phase until symptom remission and CRP normalization [1]. Return to competitive sports in uncomplicated cases is allowed 3 months after the acute attack and should be prolonged to 6 months in cases of myopericarditis [1]. The time of exercise restriction, especially in cases of recurrent forms, is a matter of debate and in general, strenuous activity should be probably even more delayed [31].
The mainstay for the medical treatment of acute pericarditis includes NSAIDS, colchicine, and proton pump inhibitors for gastroprotection. The most commonly employed NSAIDs in clinical practice are ibuprofen (600–800 mg tid), aspirin (1 g tid), naproxen (500 mg bid), and indomethacin (50 mg tid). Aspirin should be preferred in patients already receiving it for an alternative indication (e.g., coronary or peripheral artery disease). Although indomethacin has a consistent anti-inflammatory effect, concerns related to side effects (mainly gastrointestinal) limit its use especially in the elderly and in patients with coronary artery disease. NSAID dose tapering, although not supported by high-quality data, is supported by most experts [1, 7, 21]. According to the current recommendations, the full dose should be given for 7–10 days, and tapering should be performed in the following 3–4 weeks in an individualized manner (250–500 mg for aspirin and 200–400 mg for ibuprofen every 1–2 weeks). Before dose tapering, CRP should normalize; otherwise, the patient is exposed to a hazard of recurrence (2.98) [24]. In cases of recurrent pericarditis, longer duration of NSAID administration is often required [1]. Interestingly in a recent study, beta-blockers on top of standard anti-inflammatory therapies have been associated with improved symptom control through lowering of the heart rate and consequently friction between the pericardial layers [32].
Colchicine has been established as the backbone medication in the whole spectrum of pericarditis (first episode and recurrences) [1, 7, 21, 33]. It is the only medication that definitely affects recurrences, as it has been shown to halve the rate of first and subsequent relapses. It is administered on top of anti-inflammatory medications (either NSAIDs or corticosteroids). The recommended dose is 0.5–0.6 mg twice daily [1, 34]. It is a safe medication and the most common adverse effects are gastrointestinal side effects (mainly diarrhea) in up to 10% of cases [1, 7, 21, 34]. Since colchicine has a narrow therapeutic index, interaction with other medication may be worrying [7, 34, 35]. However, this is fairly unusual at the doses recommended for pericarditis. In patients aged > 70 years and those weighing < 70 kg, starting with half dose should be considered, in order to avoid side effects leading to drug discontinuation [1, 34]. Moreover, dose adjustments should be performed in chronic renal disease in relation to creatinine clearance and hepatic function [1]. Colchicine should be administered for 3 months in a first episode of acute pericarditis, and for at least 6 months in recurrent forms. Longer periods of administration should be individually considered in refractory cases [1]. Colchicine at present is not recommended as monotherapy in any pericardial syndrome and does not seem to be beneficial in the absence of overt inflammation, namely with normal CRP [1, 33]. According to a recent investigation, its usefulness with respect to recurrences has been questioned in low-risk cases (patients with a first episode not receiving glucocorticoids), but this is not enough to change the established practice of administering colchicine in all comers with acute pericarditis [35].
In pericarditis treatment algorithm, glucocorticoids constitute a second-line treatment option [1, 36]. They are administered in cases of true allergy or intolerance to NSAIDs, recent gastrointestinal ulcer, concomitant anticoagulant therapy with high risk of bleeding, chronic renal disease (NSAIDs are contraindicated in cases with creatinine clearance below 30 ml/min and should be administered with caution when clearance is between 30 and 50 ml/min), pregnancy beyond the 20th week of gestation, systemic inflammatory diseases, and possibly post-cardiac syndromes where they seem more efficacious [1, 7, 21]. Glucocorticoids are extremely effective in providing fast symptom relief. However, they may favor side effects, and their safety profile, when administered for long periods, is concerning [7]. The recommended dose is 0.2–0.5 mg of prednisone or equivalent dose of an alternative steroid, provided that bacterial infections, including tuberculosis, have been ruled out [1]. After administration of the full dose until symptom resolution and CRP normalization (usually after 1–2 weeks), dose tapering should be performed depending on the starting dose, according to the ESC guideline recommendations [1]. Notably, very slow decrements should be performed in refractory recurrent cases, especially when the dose administered is close to the threshold of the individual patient for recurrence [1]. Supplementation with calcium and vitamin D in all cases and bisphosphonates in men and postmenopausal women are recommended to restore calcium balance and bone loss, especially when long-term treatments are supposed to be given [7].
The subgroup of patients with colchicine-resistant glucocorticoid-dependent recurrent pericarditis is a field of great interest and intense research. This is a very challenging population with an average disease duration of ~ 4.7 years, needing a tailored to the individual patient treatment plan, according to the clinical phenotype [28, 37•]. A detailed report on this subgroup is out of the scope of this short update. In brief, treatment options include triple therapy with NSAIDs, colchicine, and glucocorticoids [36]. NSAIDs are usually added when relapses appear during the tapering process of glucocorticoids, in an effort to break the vicious circle of steroid dependency [1, 36]. Other options include immunomodulatory, immunosuppressant, and biological drugs including intravenous human immunoglobulins, azathioprine, hydroxychloroquine, anakinra, and rilonacept [1, 38, 39]. However, solid high-quality data are available for the latter two biological agents [40, 41, 42••, 43•]. The rationale for the introduction of anakinra and rilonacept in refractory pericarditis treatment is the emerging concept that recurrent pericarditis (or a list of a proportion of cases) belongs to the broader family of autoinflammatory disorders such as familial Mediterranean fever [37•, 44]. In these disorders, an abnormal activation of the innate immune system and IL-1 displays a central role in disease manifestations [44]. Both anakinra and rilonacept inhibit IL-1α and β, and have been proven extremely efficacious in refractory pericarditis, achieving a rapid and sustained disease remissions with a good safety profile [40, 41, 42••, 43•]. IL-1 inhibition is particularly recommended in cases characterized by a high inflammatory burden, as disclosed by CRP elevation and fever at each recurrence [37•]. In contrast, in case of a non-inflammatory phenotype, especially if the presence of an autoimmune disease, intravenous immunoglobulins and azathioprine may be beneficial [22, 37•]. An issue that needs particular attention is that although IL-1 inhibition is an important innovation in the treatment of recurrent pericarditis, further relapses may appear after discontinuation of treatment [45]. Finally, as a last resort in cases of recurrent pericarditis refractory to treatment, or in those in which treatment cannot be tolerated due to side effects, pericardiectomy should be performed in experienced referral centers [1].
In the setting of COVID-19, the currently in use medications for pericarditis (such as colchicine, glucocorticoids, and anakinra) are acceptable [46]. Concerns about the worsening of COVID-19 with NSAIDs are not supported by solid scientific evidence [46].
Prognosis
The prognosis of acute pericarditis largely depends on the underlying etiology [1]. It is excellent in cases of idiopathic pericarditis, whereas it is ominous in cases of malignant involvement of the pericardium (either in the form of primary or metastatic tumor) [1, 7]. In a Finnish registry, the mortality rate in patients hospitalized with acute pericarditis was 1.1% [3]. Complications recorded after a first episode of acute pericarditis in an Italian study with 60-month follow-up include cardiac tamponade (1.2%), constrictive pericarditis (0.48%), and recurrent pericarditis (25%). The relevant rates reported in secondary forms are 20.2% (a rate mainly driven by neoplastic pericarditis), 8.3%, and 57.1% respectively [5]. Notably, incessant pericarditis has been recently associated with constrictive pericarditis [11]. Arrhythmias, most often atrial fibrillation/flutter, develop in 4.3% of cases and the decision for chronic anticoagulation should be based on CHA2DS2-VASc score [47]. As already mentioned, periodical evaluation of the patient is mandatory, especially if new symptoms appear, in order to unveil previously unrecognized specific causes and adapt medical treatment [28, 48].
A major challenge in the management of acute pericarditis is the identification of patients prone to recurrences. An aggressive treatment of these patients is recommended with administration of the highest tolerable dose of anti-inflammatory treatment along with colchicine, serial CRP measurement before dose tapering, and eventually treatment guidance by CMR as well as recourse to novel therapies such as anti-IL-1 agents. Factors reported to be associated with recurrences include use of glucocorticoids (especially high doses with fast tapering), lack of colchicine administration, and tapering of anti-inflammatory treatment prior to CRP normalization [6•, 10]. Recently, a risk score (0–22 points) predicting recurrence in patients hospitalized with a first episode of acute pericarditis has been developed in our institution [6•]. The risk score includes 6 variables independently associated with relapses, namely age, platelet count, and effusion size as negative predictors and in-hospital use of corticosteroids, heart rate, and reduced inferior vena cava collapse as positive predictors. In patients with low score, the observed rate of recurrences was 21.3%, whereas in those with high score, the relevant rate was 69.8%. A second risk score (Torino Risk Score) has been also recently presented as a risk stratification tool to predict complicated pericarditis in patients with a first of subsequent episodes of acute pericarditis [49].
Conclusions–Perspectives
Acute pericarditis has gained attention in recent years in light of novel data regarding pathophysiological issues and the novel treatments especially for refractory cases. The COVID-19 pandemic further enhanced the public and media attention on pericardial syndromes, since pericarditis is a potential complication appearing either in the setting of COVID-19 or as a complication after vaccination against COVID-19, which may affect confidence in vaccination.
Despite the big steps forward in understanding and, subsequently, treating acute pericarditis, there is still room for further research. Towards this scope, the recent availability of animal models for pericarditis is of paramount importance for the decodification of recurrent pericarditis and possibly the identification of patients prone to recurrences, which is a major challenge in the field of pericarditis. In recent years, drugs that target the pathophysiology have been developed such as colchicine, an inhibitor of NLRP3 inflammasome formation and anti-IL-1 medications [50, 51]. In this line, additional medications able to achieve long-standing and definite remissions after discontinuation of treatment are most welcome. Even though the most recent ESC guidelines on pericardial syndromes shed more light in the hazy landscape of pericardial syndromes, they are still affected by the high rate of recommendations with level of evidence C (~ 75%) [1]. Thus, additional research is required to provide evidence-based data for the optimal management of pericardial syndromes [52].
Abbreviations
- CMR:
-
Cardiac magnetic resonance
- COVID-19:
-
Coronavirus disease-19
- CRP:
-
C-reactive protein
- CCT:
-
Cardiac computed tomography
- ESC:
-
European Society of Cardiology
- HIV:
-
Human immunodeficiency virus
- IL-1:
-
Interleukin-1
- IVIG:
-
Intravenous immunoglobulin
- LGE:
-
Late gadolinium enhancement
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- PET:
-
Positron emission tomography
- PPI:
-
Proton pump inhibitors
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Adler Y, Charron P, Imazio M, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2015;36:2921–64. https://doi.org/10.1093/eurheartj/ehv318.
Imazio M, Cecchi E, Demichelis B, et al. Myopericarditis versus viral or idiopathic acute pericarditis. Heart. 2008;94:498–501. https://doi.org/10.1136/hrt.2006.104067.
Kytö V, Sipilä J, Rautava P. Clinical profile and influences on outcomes in patients hospitalized for acute pericarditis. Circulation. 2014;130:1601–6. https://doi.org/10.1161/CIRCULATIONAHA.114.010376.
Lazaros G, Antonopoulos AS, Lazarou E, et al. Age- and sex-based differences in patients with acute pericarditis. Eur J Clin Invest. 2021;51: e13392. https://doi.org/10.1111/eci.13392.
Imazio M, Brucato A, Maestroni S, et al. Risk of constrictive pericarditis after acute pericarditis. Circulation. 2011;124:1270–5. https://doi.org/10.1161/CIRCULATIONAHA.111.018580.
• Lazarou E, Lazaros G, Antonopoulos AS, et al. A risk score for pericarditis recurrence. Eur J Clin Invest. 2021;51: e13602. https://doi.org/10.1111/eci.13602. This is the first risk score developed to predict recurrences in a patient with a first episode of recurrent pericarditis.
Imazio M, Spodick DH, Brucato A, et al. Controversial issues in the management of pericardial diseases. Circulation. 2010;121:916–28. https://doi.org/10.1161/CIRCULATIONAHA.108.844753.
Kontzias A, Barkhodari A, Yao Q. Pericarditis in systemic rheumatologic diseases. Curr Cardiol Rep. 2020;22:142. https://doi.org/10.1007/s11886-020-01415-w.
Lazaros G, Imazio M, Brucato A, Tousoulis D. Untying the Gordian knot of pericardial diseases: a pragmatic approach. Hellenic J Cardiol. 2016;57:315–22. https://doi.org/10.1016/j.hjc.2016.11.024.
Cremer PC, Kumar A, Kontzias A, et al. Complicated pericarditis: understanding risk factors and pathophysiology to inform imaging and treatment. J Am Coll Cardiol. 2016;68:2311–28. https://doi.org/10.1016/j.jacc.2016.07.785.
Andreis A, Imazio M, Giustetto C, et al. Anakinra for constrictive pericarditis associated with incessant or recurrent pericarditis. Heart. 2020;106:1561–5. https://doi.org/10.1136/heartjnl-2020-316898.
Rey F, Delhumeau-Cartier C, Meyer P, Genne D. Is acute idiopathic pericarditis associated with recent upper respiratory tract infection or gastroenteritis?. A case-control study BMJ Open. 2015;5: e009141. https://doi.org/10.1136/bmjopen-2015-009141.
Brucato A, Imazio M, Cremer PC, et al. Recurrent pericarditis: still idiopathic?. The pros and cons of a well-honoured term. Intern Emerg Med. 2018;13:839–44. https://doi.org/10.1007/s11739-018-1907-x.
Imazio M, Demichelis B, Parrini I, et al. Day-hospital treatment of acute pericarditis: a management program for outpatient therapy. J Am Coll Cardiol. 2004;43:1042–6. https://doi.org/10.1016/j.jacc.2003.09.055.
Furqan MM, Verma BR, Cremer PC, et al. Pericardial diseases in COVID19: a contemporary review. Curr Cardiol Rep. 2021;23:90. https://doi.org/10.1007/s11886-021-01519-x.
• Lazaros G, Klein AL, Hatziantoniou S, et al. The novel platform of mRNA COVID-19 vaccines and myocarditis: clues into the potential underlying mechanism. Vaccine. 2021;39:4925–7. https://doi.org/10.1016/j.vaccine.2021.07.016. In this paper they briefly summarized the potential mechanisms of inflammatory heart disease upon COVID-19 vaccination.
Buckley BJR, Harrison SL, Fazio-Eynullayeva E, et al. Prevalence and clinical outcomes of myocarditis and pericarditis in 718,365 COVID-19 patients. Eur J Clin Invest. 2021;51: e13679. https://doi.org/10.1111/eci.13679.
•• Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2021 Dec 14. https://doi.org/10.1038/s41591-021-01630-0. This paper depicts the rate of complications following SRS CoV-2 infection and vaccination against COVID-19.
Diaz GA, Parsons GT, Gering SK, et al. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021;326:1210–2. https://doi.org/10.1001/jama.2021.13443.
Lazaros G, Anastassopoulou C, Hatziantoniou S, et al. A case series of acute pericarditis following COVID-19 vaccination in the context of recent reports from Europe and the United States. Vaccine. 2021;39:6585–90. https://doi.org/10.1016/j.vaccine.2021.09.078.
Imazio M, Gaita F. Diagnosis and treatment of pericarditis. Heart. 2015;101:1159–68. https://doi.org/10.1136/heartjnl-2014-306362.
Imazio M. Noninfectious pericarditis: management challenges for cardiologists. Kardiol Pol. 2020;78:396–403. https://doi.org/10.33963/KP.15353.
Lazaros G, Solomou E, Antonopoulos AS, et al. The landscape of acute pericarditis in Greece: experience from a tertiary referral center. Hellenic J Cardiol. 2019;60:139–40. https://doi.org/10.1016/j.hjc.2018.06.011.
Imazio M, Brucato A, Maestroni S, et al. Prevalence of C-reactive protein elevation and time course of normalization in acute pericarditis: implications for the diagnosis, therapy, and prognosis of pericarditis. Circulation. 2011;123:1092–7. https://doi.org/10.1161/CIRCULATIONAHA.110.986372.
Mager A, Hammer Y, Ofek H, et al. Prognostic and diagnostic significance of serum high-sensitivity C-reactive protein level in patients with acute idiopathic pericarditis. Isr Med Assoc J. 2019;21:747–51. PMID: 31713364.
Chetrit M, Xu B, Verma BR, Klein AL. Multimodality imaging for the assessment of pericardial diseases. Curr Cardiol Rep. 2019;21:41. https://doi.org/10.1007/s11886-019-1115-y.
•• Chiabrando JG, Bonaventura A, Vecchié A, et al. Management of acute and recurrent pericarditis: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:76–92. https://doi.org/10.1016/j.jacc.2019.11.021. Comprehensive review describing the whole spectrum of complicated pericarditis.
Brucato A, Brambilla G, Moreo A, et al. Long-term outcomes in difficult-to-treat patients with recurrent pericarditis. Am J Cardiol. 2006;98:267–71. https://doi.org/10.1016/j.amjcard.2006.01.086.
Salisbury AC, Olalla-Gómez C, et al. Frequency and predictors of urgent coronary angiography in patients with acute pericarditis. Mayo Clin Proc. 2009;84:11–5. https://doi.org/10.1016/S0025-6196(11)60801-X.
Imazio M, Brucato A, Barbieri A, et al. Good prognosis for pericarditis with and without myocardial involvement: results from a multicenter, prospective cohort study. Circulation. 2013;128:42–9. https://doi.org/10.1161/CIRCULATIONAHA.113.001531.
Shah NP, Verma BR, Ala CK, et al. Exercise is good for the heart but not for the inflamed pericardium?. JACC Cardiovasc Imaging. 2019;12:1880–1. https://doi.org/10.1016/j.jcmg.2019.01.022.
Imazio M, Andreis A, Agosti A, et al. Usefulness of beta-blockers to control symptoms in patients with pericarditis. Am J Cardiol. 2021;146:115–9. https://doi.org/10.1016/j.amjcard.2021.01.032.
Imazio M, Nidorf M. Colchicine and the heart. Eur Heart J. 2021;42:2745–60.
Papageorgiou N, Briasoulis A, Lazaros G, et al. Colchicine for prevention and treatment of cardiac diseases: a meta-analysis. Cardiovasc Ther. 2017;35:10–8. https://doi.org/10.1111/1755-5922.12226.
Sambola A, Roca Luque I, et al. Colchicine administered in the first episode of acute idiopathic pericarditis: a randomized multicenter open-label study. Rev Esp Cardiol (Engl Ed). 2019;72:709–16. https://doi.org/10.1016/j.rec.2018.11.016.
Imazio M, Lazaros G, Brucato A, Gaita F. Recurrent pericarditis: new and emerging therapeutic options. Nat Rev Cardiol. 2016;13:99–105. https://doi.org/10.1038/nrcardio.2015.115.
• Tombetti E, Mulè A, Tamanini S, et al. Novel pharmacotherapies for recurrent pericarditis: current options in 2020. Curr Cardiol Rep. 2020;22:59. https://doi.org/10.1007/s11886-020-01308-y. This review describes in detail the possible clinical phenotypes in patients with recurrent pericarditis.
Lazaros G, Tousoulis D, Vassilopoulos D. Editorial commentary: Recurrent pericarditis in the era of interleukin-1 inhibition. Trends Cardiovasc Med. 2021;31:275–6. https://doi.org/10.1016/j.tcm.2020.04.010.
Lazaros G, Antonopoulos AS, Antonatou K, et al. Hydroxychloroquine for colchicine-resistant glucocorticoid-dependent idiopathic recurrent pericarditis: a pilot observational prospective study. Int J Cardiol. 2020;311:77–82. https://doi.org/10.1016/j.ijcard.2020.03.069.
Brucato A, Imazio M, Gattorno M, et al. Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence: the AIRTRIP randomized clinical trial. JAMA. 2016;316:1906–12. https://doi.org/10.1001/jama.2016.15826.
Imazio M, Andreis A, De Ferrari GM, et al. Anakinra for corticosteroid-dependent and colchicine-resistant pericarditis: the IRAP (International Registry of Anakinra for Pericarditis) study. Eur J Prev Cardiol. 2020;27:956–64. https://doi.org/10.1177/2047487319879534.
•• Klein AL, Imazio M, Cremer P, et al RHAPSODY Investigators. Phase 3 trial of interleukin-1 trap rilonacept in recurrent pericarditis. N Engl J Med. 2021;384:31–41. Doi: https://doi.org/10.1056/NEJMoa2027892. A randomized trial demonstrating the efficacy and safety of rilonacept in recurrent pericarditis.
• Imazio M, Lazaros G, Gattorno M, et al. Anti-interleukin-1 agents for pericarditis: a primer for cardiologists. Eur Heart J. 2021 Sep 16:ehab452. doi: https://doi.org/10.1093/eurheartj/ehab452. A concise review on the indications and use of IL-1 inhibitors in pericarditis.
Lazaros G, Antonatou K, Vassilopoulos D. The therapeutic role of interleukin-1 inhibition in idiopathic recurrent pericarditis: current evidence and future challenges. Front Med (Lausanne). 2017;4:78. https://doi.org/10.3389/fmed.2017.00078.
Lazaros G, Tsioufis K, Vassilopoulos D. Phase 3 trial of interleukin-1 trap rilonacept in recurrent pericarditis. N Engl J Med. 2021;384:1474–5. https://doi.org/10.1016/j.euo.2021.04.010.
Imazio M, Brucato A, Lazaros G, et al. Anti-inflammatory therapies for pericardial diseases in the COVID-19 pandemic: safety and potentiality. J Cardiovasc Med (Hagerstown). 2020;21:625–9. https://doi.org/10.2459/JCM.0000000000001059.
Imazio M, Lazaros G, Picardi E, et al. Incidence and prognostic significance of new onset atrial fibrillation/flutter in acute pericarditis. Heart. 2015;101:1463–7. https://doi.org/10.1136/heartjnl-2014-307398.
Lazaros G, Vlachopoulos C, Lazarou E, et al. Acute idiopathic pericarditis: is it actually always idiopathic? J Am Coll Cardiol. 2021;77:1484–5. https://doi.org/10.1016/j.jacc.2020.12.064.
Imazio M, Andreis A, Lubian M, et al. The Torino Pericarditis Score: a new-risk stratification tool to predict complicated pericarditis. Intern Emerg Med. 2021;16:1921–6. https://doi.org/10.1007/s11739-021-02803-y.
Vecchié A, Del Buono MG, Chiabrando GJ, Dentali F, Abbate A, Bonaventura A. Interleukin-1 and the NLRP3 inflammasome in pericardial disease. Curr Cardiol Rep. 2021;23:157. https://doi.org/10.1007/s11886-021-01589-x.
Imazio M, Andreis A, Piroli F, et al. Anti-interleukin 1 agents for the treatment of recurrent pericarditis: a systematic review and meta-analysis. Heart. 2021 Mar 18:heartjnl-2020–318869. Doi: https://doi.org/10.1136/heartjnl-2020-318869.
Lazaros G, Aznaouridis K, Lazarou E, et al. The prognostic impact of the 2015 European Society of Cardiology pericarditis guidelines implementation in clinical practice. Hellenic J Cardiol. 2021;S1109–9666(21):00187–91. https://doi.org/10.1016/j.hjc.2021.10.006.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Lazaros received a travel grant and advisory board compensation from Kiniksa Pharmaceuticals Ltd. The rest of the authors declare that they have no conflict of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pericardial Disease
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lazarou, E., Tsioufis, P., Vlachopoulos, C. et al. Acute Pericarditis: Update. Curr Cardiol Rep 24, 905–913 (2022). https://doi.org/10.1007/s11886-022-01710-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-022-01710-8