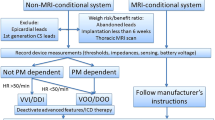
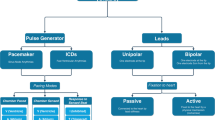
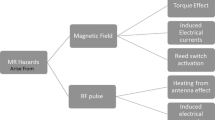
Abstract
Magnetic resonance imaging (MRI) has evolved into an essential diagnostic modality for the evaluation of various conditions. In line with the increase in MRI applications, the use of cardiac implantable electronic devices (CIED) is growing and many of the CEID recipients of today may require MRI examinations in the future. Traditionally, MRI examination of CIED recipients has been considered a contraindication. However, recent studies have provided strong evidence that MRI can safely be performed in selected patients with specific precautions. This review highlights the interactions of MRI with CIEDs, summarizes the literature, and outlines the Johns Hopkins Safety Protocol.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009—a World Society of Arrhythmia’s Project. Pacing Clin Electrophysiol. 2011;34:1013–27.
Kalin R, Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol: PACE. 2005;28:326–8.
Martin ET, Coman JA, Shellock FG, et al. Magnetic resonance imaging and cardiac pacemaker safety at 1.5-Tesla. J Am Coll Cardiol. 2004;43:1315–24.
Roguin A, Zviman MM, Meininger GR, et al. Modern pacemaker and implantable cardioverter/defibrillator systems can be magnetic resonance imaging safe: in vitro and in vivo assessment of safety and function at 1.5 T. Circulation. 2004;110:475–82.
Nazarian S, Roguin A, Zviman MM, et al. Clinical utility and safety of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable cardioverter defibrillators at 1.5 Tesla. Circulation. 2006;114:1277–84.
Levine GN, Gomes AS, Arai AE, et al. Safety of magnetic resonance imaging in patients with cardiovascular devices. Circulation. 2007;116:2878–91.
Fetter J, Aram G, Holmes Jr DR, et al. The effects of nuclear magnetic resonance imagers on external and implantable pulse generators. Pacing Clin Electrophysiol. 1984;7(4):720–7.
Nazarian S, Halperin HR. How to perform magnetic resonance imaging on patients with implantable cardiac arrhythmia devices. Heart Rhythm. 2009;6:138–43.
Luechinger R, Duru F, Scheidegger MB, et al. Force and torque effects of a 1.5 Tesla MRI scanner on cardiac pacemakers and ICD’s. Pacing Clin Electrophysiol. 2001;24:199–205.
Luechinger R, Zeijlemaker VA, Pederson EM, et al. In vivo heating of pacemaker leads during magnetic resonance imaging. Eur Heart J. 2004;26:376–83.
Luechinger R, Duru F, Zeijlemaker VA, et al. Pacemaker reed switch behavior in 0.5, 1.5, and 3.0 Tesla magnetic resonance imaging units: are reed switches always closed in strong magnetic fields? Pacing Clin Electrophysiol. 2002;25:1419–23.
Nazarian S, Hansford R, Roguin A, et al. A prospective evaluation of a protocol for magnetic resonance imaging of patients with implanted cardiac devices. Ann Intern Med. 2011;155:415–24.
Coman JA, Martin ET, Sandler DA, et al. Implantable cardiac defibrillator interactions with magnetic resonance imaging at 1.5 Tesla. J Am Coll Cardiol. 2004;43:138A.
Nazarian S, Beinart R, Halperin HR. Magnetic resonance imaging and implantable devices. Circ Arrhythm Electrophysiol. 2013;6(2):419–28.
Ainslie M, Miller C, Brown B, et al. Cardiac MRI of patients with implanted electrical cardiac devices. Heart. 2013;0:1–7.
Gimbel JR, Johnson D, Levine PA, et al. Safe performance of magnetic resonance imaging on five patients with permanent cardiac pacemakers. Pacing Clin Electrophysiol. 1996;19(6):913–9.
Fontaine JM, Mohamed FB, Gottlieb C, et al. Rapid ventricular pacing in a pacemaker patient undergoing magnetic resonance imaging. Pacing Clin Electrophysiol. 1998;21:1336–9.
Sommer T, Vahlhaus C, Lauck G, et al. MRI and cardiac pacemakers: in-vitro evaluation and in vivo studies in 51 patients at 0.5 T. Radiology. 2000;215:869–79.
Vahlhaus C, Sommer T, Lewalter T, et al. Interference with cardiac pacemakers by magnetic resonance imaging: are there irreversible changes at 0.5 T? Pacing Clin Electrophysiol. 2001;24:489–95.
Gimbel JR, Bailey SM, Tchou PJ, et al. Strategies for the safe magnetic resonance imaging of pacemaker-dependent patients. Pacing Clin Electrophysiol. 2005;28:1041–6.
Del Ojo JL, Moya F, Villalba J, et al. Is magnetic resonance imaging safe in cardiac pacemaker recipients? Pacing Clin Electrophysiol. 2005;28:274–8.
Kaasalainen T, Pakarinen S, Kivistö S, et al. MRI with cardiac pacing devices—safety in clinical practice. Eur J Radiol. 2014;83(8):1387–95. This is one of the most recent studies that evaluated the safety results of both MRI conditional and MRI unsafe devices in clinical practice. Out of 64 patients, 22 had MRI conditional CIED. There were no MRI related complications or clinically significant changes in system parameters following MRI examination.
Naehle CP, Strach K, Thomas D, et al. Magnetic resonance imaging at 1.5-T in patients with implantable cardioverter-defibrillators. J Am Coll Cardiol. 2009;54(6):549–55.
Mollerus M, Albin G, Lipinski M, et al. Magnetic resonance imaging of pacemakers and implantable cardioverter-defibrillators without specific absorption rate restrictions. Europace. 2010;12(7):947–51.
Junttila MJ, Fishman JE, Lopera GA, et al. Safety of serial MRI in patients with implantable cardioverter defibrillators. Heart. 2011;97:1852–6.
Zikria JF, Machnicki S, Rhim E, et al. MRI of patients with cardiac pacemakers: a review of the medical literature. Am J Roentgenol. 2011;196:390–401.
Cohen JD, Costa HS, Russo RJ. Determining the risks of magnetic resonance imaging at 1.5 Tesla for patients with pacemakers and implantable cardioverter defibrillators. Am J Cardiol. 2012;110:1631–6. This study is the first to use control group who had CIED and did not undergo MRI. Interestingly, there were no significant difference between groups that may indicate that minor changes in system parameters may be due to MRI related interaction, or may reflect normal variation/fluctuation over time. But one major limitation was the retrospective design of the study.
Russo RJ. Determining the risks of clinically indicated nonthoracic magnetic resonance imaging at 1.5 T for patients with pacemakers and implantable cardioverter-defibrillators: rationale and design of the MagnaSafe Registry. Am Heart J. 2013;165:266–72. MagnaSafe Registry is an ongoing randomized trial that targets to perform 1500 MRI to non-MRI conditional CIED recipients. As of 2013, 1189 patients were enrolled. So far no deaths or severe complication were reported. However, clinically relevant device parameter changes and partial electrical resets have been observed in minority of the cases. This study will be completed in the near future and will add more information in regard to MRI safety in patients with non-MRI conditional CIED.
Roguin A, Schwitter J, Vahlhaus C, et al. Magnetic resonance imaging in individuals with cardiovascular implantable electronic devices. Europace. 2008;10:336–46.
Kanal E, Barkovich AJ, Bell C, et al. ACR Blue Ribbon Panel on MR safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188:1447–74.
Wilkoff BL, Bello D, Taborsky M, et al. Magnetic resonance imaging in patients with a pacemaker system designed for the magnetic resonance environment. Heart Rhythm. 2011;8:65–73.
Forleo GB, Santini L, Della Rocca DG, et al. Safety and efficacy of a new magnetic resonance imaging-compatible pacing system: early results of a prospective comparison with conventional dual- chamber implant outcomes. Heart Rhythm. 2010;7:750–4.
Gimbel JR, Bello D, Schmitt M, et al. Randomized trial of pacemaker and lead system for safe scanning at 1.5 Tesla. Heart Rhythm. 2013;10:685–91. In this prospective randomized multicenter trial, another MRI conditional device was proved to be safe for 1.5 T MRI. Due to favorable results of this trial, Advisa SureScan MRI pacemaker system was approved by FDA.
Wollmann CG, Thudt K, Kaiser B, et al. Safe performance of magnetic resonance of the heart in patients with magnetic resonance conditional pacemaker systems: the safety issue of the ESTIMATE study. J Cardiovasc Magn Reson. 2014;16:30. This single center study demonstrated that 1.5 T cardiac MRI is safe for MRI conditional devices. During short term and long term follow-up, there were only minor changes in system parameters.
Langman DA, Goldberg IB, Finn JP, et al. Pacemaker lead tip heating in abandoned and pacemaker-attached leads at 1.5 Tesla MRI. J Magn Reson Imaging. 2011;33:426–31.
Sommer T, Naehle CP, Yang A, et al. Strategy for safe performance of extrathoracic magnetic resonance imaging at 1.5 Tesla in the presence of cardiac pacemakers in non-pacemaker-dependent patients: a prospective study with 115 examinations. Circulation. 2006;114:1285–92.
Higgins JV, Gard JJ, Sheldon SH, et al. Safety and outcomes of magnetic resonance imaging in patients with abandoned pacemaker and defibrillator leads. Pacing Clin Electrophysiol. 2014;37(10):1284–90. This study retrospectively evaluated the impact of MRI on retained leads. There were no MRI-related clinical events; although the absence of the tissue damage was not histopathologically confirmed. Out of 19 patients, 12 had their leads reconnected to a generator after MRI examination; in this subgroup of cases, lead parameters did not show any clinically significant difference.
Acknowledgments
Saman Nazarian is supported by NIH Grants K23HL089333 and R01HL116280.
The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Dr. Esra Gucuk Ipek declares that she has no conflict of interest.
Dr. Saman Nazarian is PI for research funding to Johns Hopkins University from Biosense Webster Inc. and is also a scientific advisor to Biosense Webster Inc. and Medtronic Inc.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Invasive Electrophysiology and Pacing
Rights and permissions
About this article
Cite this article
Ipek, E.G., Nazarian, S. Safety of Implanted Cardiac Devices in an MRI Environment. Curr Cardiol Rep 17, 51 (2015). https://doi.org/10.1007/s11886-015-0605-9
Published:
DOI: https://doi.org/10.1007/s11886-015-0605-9