Abstract
Purpose of Review
The purpose of the review is to outline current guidelines for evaluation and treatment of nocturia.
Recent Findings
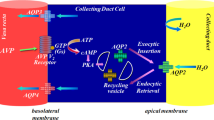
Nocturnal polyuria currently has no consensus definition; it is most often reported as either nocturnal urine production (NUP) > 90 mL/h or nocturnal polyuria index (NPi) > .33. Clinical trials for oral dispersible desmopressin (melt) demonstrate utility as a pharmacological treatment of refractory nocturnal polyuria, but also introduce risk of hyponatremia; it currently is unapproved in the USA.
Summary
Nocturia is a prevalent condition that shows an increased risk with age and is associated with increased mortality and decreased quality of life. Nocturia should be managed by first classifying the cause as either global polyuria, nocturnal polyuria, diminished bladder capacity, sleep disorder, or mixed etiology through bladder diaries, and then further specifying the etiology. Effective nocturia treatment is dependent on its cause and should be determined on a case-by-case basis. Treatment recommendations include addressing comorbidities, behavioral therapy, pharmacological therapy, and surgical intervention.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
van Kerrebroeck P, Abrams P, Chaikin D, Donovan J, Fonda D, Jackson S, et al. The standardisation of terminology in nocturia: report from the standardisation sub‐committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):179–83.
Bosch JR, Weiss JP. The prevalence and causes of nocturia. J Urol. 2010;184(2):440–6.
Vaughan CP, Eisenstein R, Bliwise DL, Endeshaw YK, Nagamia ZJ, Wolf RA, et al. Self‐rated sleep characteristics and bother from nocturia. Int J Clin Pract. 2012;66(4):369–73.
•• Häkkinen JT, Hakama M, Shiri R, Auvinen A, Tammela TL, Koskimäki J. Incidence of nocturia in 50 to 80-year-old Finnish men. J Urol. 2006;176(6):2541–5. The FINNO study is the most important cross sectional epidemiological study available on the topic of nocturia.
Hernández C, Estivill E, Prieto M, Badía X. Nocturia in Spanish patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia (LUTS/BPH). Curr Med Res Opin. 2008;24(4):1033–8.
Tikkinen KA, Johnson TM, Tammela TL, Sintonen H, Haukka J, Huhtala H, et al. Nocturia frequency, bother, and quality of life: how often is too often? A population-based study in Finland. Eur Urol. 2010;57(3):488–98.
Kupelian V, Fitzgerald MP, Kaplan SA, Norgaard JP, Chiu GR, Rosen RC. Association of nocturia and mortality: results from the third national health and nutrition examination survey. J Urol. 2011;185(2):571–7.
Lightner DJ, Krambeck AE, Jacobson DJ, McGree ME, Jacobsen SJ, Lieber MM, et al. Nocturia is associated with an increased risk of coronary heart disease and death. BJU Int. 2012;110(6):848–53.
Beers TM. Flexible schedules and shift work: replacing the 9-to-5 workday. Mon Labor Rev. 2000;123:33.
Bliwise DL, Holm-Larsen T, Goble S, Nørgaard JP. Short time to first void is associated with lower whole-night sleep quality in nocturia patients. J Clin Sleep Med: JCSM: Off Publ Am Acad Sleep Med. 2015;11(1):53–5.
Homma Y, Yamaguchi O, Kageyama S, Nishizawa O, Yoshida M, Kawabe K. Nocturia in the adult: classification on the basis of largest voided volume and nocturnal urine production. J Urol. 2000;163(3):777–81.
• van Doorn B, Blanker MH, Kok ET, Westers P, Bosch JR. Prevalence, incidence, and resolution of nocturnal polyuria in a longitudinal community-based study in older men: the Krimpen study. Eur Urol. 2013;63(3):542–7. The Krimpen study is a uniquely longitudinal epidemiological study of nocturia utilizing voiding diaries.
Oelke M, van Kerrebroeck P, Cartwright R. Current aspects on diagnosis and treatment of nocturia. Bremen: UNI-MED; 2012.
Baruzzi A, Riva R, Cirignotta F, Zucconi M, Cappelli M, Lugaresi E. Atrial natriuretic peptide and catecholamines in obstructive sleep apnea syndrome. Sleep. 1991;14(1):83–6.
Espiner EA, Richards AM, Yandle TG, Nicholls MG. Natriuretic hormones. Endocrinol Metab Clin N Am. 1995;24(3):481–509.
Hashim H, Coyne K, Chapple C, Milsom I, Kopp Z. 884 nocturia: risk factors and associated comorbid conditions findings from an international cross-sectional study: EPILUTS. Eur Urol Suppl. 2011;10(2):278.
Guilleminault C, Lin CM, Goncalves MA, Ramos E. A prospective study of nocturia and the quality of life of elderly patients with obstructive sleep apnea or sleep onset insomnia. J Psychosom Res. 2004;56(5):511–5.
Reynard JM, Cannon A, Yang Q, Abrams P. A novel therapy for nocturnal polyuria: a double-blind randomized trial of frusemide against placebo. Br J Urol. 1998;81:215–8.
Tyagi S, Resnick NM, Perera S, Monk TH, Hall MH, Buysse DJ. Behavioral treatment of insomnia: also effective for nocturia. J Am Geriatr Soc. 2014;62(1):54–60.
Tomasi PA, Siracusano S, Monni AM, Mela G, Delitala G. Decreased nocturnal urinary antidiuretic hormone excretion in enuresis is increased by imipramine. BJU Int. 2001;88(9):932–7.
Giardina EG, Bigger JT, Glassman AH, Perel JM, Kantor SJ. The electrocardiographic and antiarrhythmic effects of imipramine hydrochloride at therapeutic plasma concentrations. Circulation. 1979;60(5):1045–52.
Swanson JR, Jones GR, Krasselt W, Denmark LN, Ratti F. Death of two subjects due to imipramine and desipramine metabolite accumulation during chronic therapy: a review of the literature and possible mechanisms. J Forensic Sci. 1997;42(2):335–9.
Sands JM. Molecular mechanisms of urea transport. J Membr Biol. 2003;191(3):149–63.
Weiss JP, Zinner NR, Klein BM, Nørgaard JP. Desmopressin orally disintegrating tablet effectively reduces nocturia: results of a randomized, double‐blind, placebo‐controlled trial. Neurourol Urodyn. 2012;31(4):441–7.
Sand PK, Dmochowski RR, Reddy J, van der Meulen EA. Efficacy and safety of low dose desmopressin orally disintegrating tablet in women with nocturia: results of a multicenter, randomized, double-blind, placebo controlled, parallel group study. J Urol. 2013;190(3):958–64.
Weiss JP, Jumadilova Z, Johnson TM, FitzGerald MP, Carlsson M, Martire DL, et al. Efficacy and safety of flexible dose fesoterodine in men and women with overactive bladder symptoms including nocturnal urinary urgency. J Urol. 2013;189(4):1396–401.
Rembratt A, Riis A, Norgaard JP. Desmopressin treatment in nocturia; an analysis of risk factors for hyponatremia. Neurourol Urodyn. 2006;25(2):105–9.
Ferring Pharmaceuticals. Desmopressin orally disintegrating tablet for the treatment of nocturia due to nocturnal polyuria in adults [Internet]. 2014;15. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM429354.pdf
Weiss JP. Nocturia: causes, consequences and clinical approaches. In: van Kerrebroeck P, Blaivas JG, Wein AJ, editors. Springer; 2012.
Cumming JA, Chisholm GD. Changes in detrusor innervation with relief of outflow tract obstruction. Br J Urol. 1992;69(1):7–11.
Housami F, Abrams P. Persistent detrusor overactivity after transurethral resection of the prostate. Curr Urol Rep. 2008;9(4):284–90.
Margel D, Lifshitz D, Brown N, Lask D, Livne PM, Tal R. Predictors of nocturia quality of life before and shortly after prostatectomy. Urology. 2007;70(3):493–7.
Antunes AA, Srougi M, Coelho RF, Leite KR, Freire GD. Transurethral resection of the prostate for the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia: how much should be resected? Int Braz J Urol. 2009;35(6):683–91.
Van Dijk MM, Wijkstra H, Debruyne FM, De La Rosette JJ, Michel MC. The role of nocturia in the quality of life of men with lower urinary tract symptoms. BJU Int. 2010;105(8):1141–6.
Platz EA, Smit E, Curhan GC, Nyberg LM, Giovannucci E. Prevalence of and racial/ethnic variation in lower urinary tract symptoms and noncancer prostate surgery in US men. Urology. 2002;59(6):877–83.
Simaioforidis V, Papatsoris AG, Chrisofos M, Chrisafis M, Koritsiadis S, Deliveliotis C. Tamsulosin versus transurethral resection of the prostate: effect on nocturia as a result of benign prostatic hyperplasia. Int J Urol. 2011;18(3):243–8.
Yoshimura K, Ohara H, Ichioka K, Terada N, Matsui Y, Terai A, et al. Nocturia and benign prostatic hyperplasia. Urology. 2003;61(4):786–90.
Johnson TM, Jones K, Williford WO, Kutner MH, Issa MM, Lepor H. Changes in nocturia from medical treatment of benign prostatic hyperplasia: secondary analysis of the department of veterans affairs cooperative study trial. J Urol. 2003;170(1):145–8.
Johnson TM, Burrows PK, Kusek JW, Nyberg LM, Tenover JL, Lepor H, et al. Medical therapy of prostatic symptoms research group. The effect of doxazosin, Finasteride and combination therapy on nocturia in men with benign prostatic hyperplasia. J Urol. 2007;178(5):2045–51.
Yokoyama O, Yamaguchi O, Kakizaki H, Itoh N, Yokota T, Okada H, et al. Efficacy of solifenacin on nocturia in Japanese patients with overactive bladder: impact on sleep evaluated by bladder diary. J Urol. 2011;186(1):170–4.
Rackley R, Weiss JP, Rovner ES, Wang JT, Guan Z, STUDY GROUP. Nighttime dosing with tolterodine reduces overactive bladder-related nocturnal micturitions in patients with overactive bladder and nocturia. Urology. 2006;67(4):731–6.
Rudy D, Cline K, Harris R, Goldberg K, Dmochowski R. Multicenter phase III trial studying trospium chloride in patients with overactive bladder. Urology. 2006;67(2):275–80.
Dmochowski RR, Peters KM, Morrow JD, Guan Z, Gong J, Sun F, et al. Randomized, double-blind, placebo-controlled trial of flexible-dose fesoterodine in subjects with overactive bladder. Urology. 2010;75(1):62–8.
Herschorn S, Swift S, Guan Z, Carlsson M, Morrow JD, Brodsky M, et al. Comparison of fesoterodine and tolterodine extended release for the treatment of overactive bladder: a head‐to‐head placebo‐controlled trial. BJU Int. 2010;105(1):58–66.
Nitti VW, Dmochowski R, Sand PK, Forst HT, Haag-Molkenteller C, Massow U, et al. Efficacy, safety and tolerability of fesoterodine for overactive bladder syndrome. J Urol. 2007;178(6):2488–94.
Weiss JP, Herschorn S, Albei CD, van der Meulen EA. Efficacy and safety of low dose desmopressin orally disintegrating tablet in men with nocturia: results of a multicenter, randomized, double-blind, placebo controlled, parallel group study. J Urol. 2013;190(3):965–72.
Nakanishi S. Efficacy of mirabegron, a β3-adrenergic agonist, switched from an anticholinergic agent for female patients aged over 70 years. Hinyokika kiyo Acta Urol Jpn. 2013;59(9):561–4.
Johnson TM, Markland AD, Goode PS, Vaughan CP, Colli JL, Ouslander JG, et al. Efficacy of adding behavioural treatment or antimuscarinic drug therapy to α‐blocker therapy in men with nocturia. BJU Int. 2013;112(1):100–8.
Weiss JP, Blaivas JG, Stember DS, Brooks MM. Nocturia in adults: etiology. Neurourol Urodyn. 1998;17:467–72.
•• Vaughan CP, Endeshaw Y, Nagamia Z, Ouslander JG, Johnson TM. A multicomponent behavioural and drug intervention for nocturia in elderly men: rationale and pilot results. BJU Int. 2009;104(1):69–74. One of few studies on outcomes of nocturia therapy outside pharma-based clinical trials.
Homma Y. Lower urinary tract symptomatology: its definition and confusion. Int J Urol. 2008;15(1):35–43.
Dundas B, Harris M, Narasimhan M. Psychogenic polydipsia review: etiology, differential, and treatment. Curr Psychiatry Rep. 2007;9(3):236–41.
Shapiro M, Weiss JP. Diabetes insipidus: a review. J Diabetes Metab. 2013;7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Drs. Epstein and Gerber declare that they have no conflict of interest.
Dr. Blaivas declares association with Symptelligence.
Dr. Weiss reports personal fees from Ferring, personal fees from Pfizer, personal fees from Allergan, personal fees from Elsevier, personal fees from Astellas, outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Overactive Bladder
Rights and permissions
About this article
Cite this article
Epstein, M., Gerber, J.A., Weiss, J.P. et al. Nocturia: Evaluation and Management. Curr Bladder Dysfunct Rep 12, 95–103 (2017). https://doi.org/10.1007/s11884-017-0412-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11884-017-0412-9