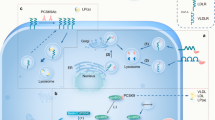
Abstract
Purpose of Review
The focus of this article is to highlight the importance of the small GTPase, Ras-associated protein 1 (Rap1), in proprotein convertase subtilisin/kexin type 9 (PCSK9) regulation and atherosclerosis and type 2 diabetes etiology and discuss the potential therapeutic implications of targeting Rap1 in these disease areas.
Review Findings
Cardiometabolic disease characterized by obesity, glucose intolerance, dyslipidemia, and atherosclerotic cardiovascular disease remain an important cause of mortality. Evidence using mouse models of obesity and insulin resistance indicates that Rap1 deficiency increases proatherogenic PCSK9 and low-density lipoprotein cholesterol levels and predisposes these mice to develop obesity- and statin-induced hyperglycemia, which highlights Rap1’s role in cardiometabolic dysfunction. Rap1 may also contribute to cardiovascular disease through its effects on vascular wall cells involved in the atherosclerosis progression.
Summary
Rap1 activation, specifically in the liver, could be beneficial in the prevention of cardiometabolic perturbations, including type 2 diabetes, hypercholesterolemia, and atherosclerosis.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mozaffarian D, Benjamin EJ, Go AS, et al. Executive summary: Heart disease and stroke statistics-2016 update A report from the American Heart Association. Circulation. 2016;133(4):447–54.
King GL, Park K, Li Q. Selective insulin resistance and the development of cardiovascular diseases in diabetes: The 2015 Edwin Bierman Award lecture. Diabetes. 2016;65(6):1462–71.
Beckman JA, Creager MA. Vascular complications of diabetes. Circ Res. 2016;118(11):1771–85.
Ference BA, Robinson JG, Brook RD, et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med. 2016;375(22):2144–53.
Brandts J, Ray KK. Novel and future lipid-modulating therapies for the prevention of cardiovascular disease. Nat Rev Cardiol. 2023;20(9):600–16.
Taylor SI, Yazdi ZS, Beitelshees AL. Pharmacological treatment of hyperglycemia in type 2 diabetes. J Clin Invest. 2021:131(2):e142243. https://doi.org/10.1172/JCI142243.
Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843.
Gloerich M, Bos JL. Regulating Rap small G-proteins in time and space. Trends Cell Biol. 2011;21(10):615–23.
Jaskiewicz A, Pajak B, Orzechowski A. The many faces of Rap1 GTPase. Int J Mol Sci. 2018;19(10):2848. https://doi.org/10.3390/ijms19102848.
Shah S, Brock EJ, Ji K, Mattingly RR. Ras and Rap1: A tale of two GTPases. Semin Cancer Biol. 2019;54:29–39.
Wittchen ES, Aghajanian A, Burridge K. Isoform-specific differences between Rap1A and Rap1B GTPases in the formation of endothelial cell junctions. Small GTPases. 2011;2(2):65–76.
Caron E. Cellular functions of the Rap1 GTP-binding protein: A pattern emerges. J Cell Sci. 2003;116(Pt 3):435–40.
Li Y, Yan J, De P, et al. Rap1a null mice have altered myeloid cell functions suggesting distinct roles for the closely related Rap1a and 1b proteins. J Immunol. 2007;179(12):8322–31.
Chrzanowska-Wodnicka M, Smyth SS, Schoenwaelder SM, Fischer TH, White GC 2nd. Rap1b is required for normal platelet function and hemostasis in mice. J Clin Invest. 2005;115(3):680–7.
Frische EW, Zwartkruis FJ. Rap1, a mercenary among the Ras-like GTPases. Dev Biol. 2010;340(1):1–9.
Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: Critical elements in the control of small G proteins. Cell. 2007;129(5):865–77.
de Rooij J, Zwartkruis FJ, Verheijen MH, et al. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396(6710):474–7.
Sartre C, Peurois F, Ley M, et al. Membranes prime the RapGEF EPAC1 to transduce cAMP signaling. Nat Commun. 2023;14(1):4157.
de Rooij J, Boenink NM, van Triest M, Cool RH, Wittinghofer A, Bos JL. PDZ-GEF1, a guanine nucleotide exchange factor specific for Rap1 and Rap2. J Biol Chem. 1999;274(53):38125–30.
Gotoh T, Hattori S, Nakamura S, et al. Identification of Rap1 as a target for the Crk SH3 domain-binding guanine nucleotide-releasing factor C3G. Mol Cell Biol. 1995;15(12):6746–53.
Crittenden JR, Bergmeier W, Zhang Y, et al. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat Med. 2004;10(9):982–6.
Lezoualc’h F, Fazal L, Laudette M, Conte C. Cyclic AMP sensor EPAC proteins and their role in cardiovascular function and disease. Circ Res. 2016;118(5):881–97.
Rubinfeld B, Munemitsu S, Clark R, et al. Molecular cloning of a GTPase activating protein specific for the Krev-1 protein p21rap1. Cell. 1991;65(6):1033–42.
Kurachi H, Wada Y, Tsukamoto N, et al. Human SPA-1 gene product selectively expressed in lymphoid tissues is a specific GTPase-activating protein for Rap1 and Rap2. Segregate expression profiles from a rap1GAP gene product. J Biol Chem. 1997;272(44):28081–8.
Pannekoek WJ, Vliem MJ, Bos JL. Multiple Rap1 effectors control Epac1-mediated tightening of endothelial junctions. Small GTPases. 2020;11(5):346–53.
Onodera Y, Nam JM, Bissell MJ. Increased sugar uptake promotes oncogenesis via EPAC/RAP1 and O-GlcNAc pathways. J Clin Invest. 2014;124(1):367–84.
Sayyah J, Bartakova A, Nogal N, Quilliam LA, Stupack DG, Brown JH. The Ras-related protein, Rap1A, mediates thrombin-stimulated, integrin-dependent glioblastoma cell proliferation and tumor growth. J Biol Chem. 2014;289(25):17689–98.
Itoh M, Nelson CM, Myers CA, Bissell MJ. Rap1 integrates tissue polarity, lumen formation, and tumorigenic potential in human breast epithelial cells. Cancer Res. 2007;67(10):4759–66.
Lyle KS, Raaijmakers JH, Bruinsma W, Bos JL, de Rooij J. cAMP-induced Epac-Rap activation inhibits epithelial cell migration by modulating focal adhesion and leading edge dynamics. Cell Signal. 2008;20(6):1104–16.
Valles AM, Beuvin M, Boyer B. Activation of Rac1 by paxillin-Crk-DOCK180 signaling complex is antagonized by Rap1 in migrating NBT-II cells. J Biol Chem. 2004;279(43):44490–6.
Looi CK, Hii LW, Ngai SC, Leong CO, Mai CW. The role of Ras-associated protein 1 (Rap1) in cancer: Bad actor or good player? Biomedicines. 2020;8(9).
Shapiro MD, Tavori H, Fazio S. PCSK9: From basic science discoveries to clinical trials. Circ Res. 2018;122(10):1420–38.
Leander K, Malarstig A, Van’t Hooft FM, et al. Circulating proprotein convertase subtilisin/kexin type 9 (PCSK9) predicts future risk of cardiovascular events independently of established risk factors. Circulation. 2016;133(13):1230–9.
Abifadel M, Varret M, Rabes JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34(2):154–6.
Horton JD, Cohen JC, Hobbs HH. Molecular biology of PCSK9: Its role in LDL metabolism. Trends Biochem Sci. 2007;32(2):71–7.
Sun H, Krauss RM, Chang JT, Teng BB. PCSK9 deficiency reduces atherosclerosis, apolipoprotein B secretion, and endothelial dysfunction. J Lipid Res. 2018;59(2):207–23.
Karagiannis AD, Liu M, Toth PP, et al. Pleiotropic anti-atherosclerotic effects of PCSK9 inhibitors from molecular biology to clinical translation. Curr Atheroscler Rep. 2018;20(4):20.
Momtazi-Borojeni AA, Sabouri-Rad S, Gotto AM, et al. PCSK9 and inflammation: A review of experimental and clinical evidence. Eur Heart J Cardiovasc Pharmacother. 2019;5(4):237–45.
Katsuki S, Kumar Jha P, Lupieri A, et al. Proprotein convertase subtilisin/kexin 9 promotes macrophage activation via LDL receptor-independent mechanisms. Circ Res. 2022;131(11):873–89. https://doi.org/10.1161/CIRCRESAHA.121.320056.
Arsenault BJ, Petrides F, Tabet F, et al. Effect of atorvastatin, cholesterol ester transfer protein inhibition, and diabetes mellitus on circulating proprotein subtilisin kexin type 9 and lipoprotein(a) levels in patients at high cardiovascular risk. J Clin Lipidol. 2018;12(1):130–6.
•• Spolitu S, Okamoto H, Dai W, et al. Hepatic glucagon signaling regulates PCSK9 and low-density lipoprotein cholesterol. Circ Res. 2019;124(1):38–51. This work has shown that Rap1 activation in the liver enhances lysosomal degradation of PCSK9 and contributes to plasma LDL cholesterol regulation.
Spolitu S, Dai W, Zadroga JA, Ozcan L. Proprotein convertase subtilisin/kexin type 9 and lipid metabolism. Curr Opin Lipidol. 2019;30(3):186–91.
Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 2011;14(1):9–19.
Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest. 1992;90(4):1323–7.
White MF, Kahn CR. Insulin action at a molecular level - 100 years of progress. Mol Metab. 2021;52:101304.
Rothman DL, Magnusson I, Katz LD, Shulman RG, Shulman GI. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science. 1991;254(5031):573–6.
Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC. Contributions of gluconeogenesis to glucose production in the fasted state. J Clin Invest. 1996;98(2):378–85.
Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol. 2017;13(10):572–87.
Cullen KA, McCool J, Anwer MS, Webster CR. Activation of cAMP-guanine exchange factor confers PKA-independent protection from hepatocyte apoptosis. Am J Physiol Gastrointest Liver Physiol. 2004;287(2):G334-343.
Gaudy AM, Clementi AH, Campbell JS, Smrcka AV, Mooney RA. Suppressor of cytokine signaling-3 is a glucagon-inducible inhibitor of PKA activity and gluconeogenic gene expression in hepatocytes. J Biol Chem. 2010;285(53):41356–65.
•• Wang Y, Spolitu S, Zadroga JA, Sarecha AK, Ozcan L: Hepatocyte Rap1a contributes to obesity- and statin-associated hyperglycemia. Cell Rep. 2022;40(8):111259. This work identified hepatic Rap1 inhibition as a mechanism involved in obesity- and statin-mediated glucose intolerance and hyperglycemia.
Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for prevention of cardiovascular disease in adults: Evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316(19):2008–24.
Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet. 2010;375(9716):735–42.
Casula M, Mozzanica F, Scotti L, et al. Statin use and risk of new-onset diabetes: A meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. 2017;27(5):396–406.
Crandall JP, Mather K, Rajpathak SN, et al. Statin use and risk of developing diabetes: Results from the Diabetes Prevention Program. BMJ Open Diabetes Res Care. 2017;5(1):e000438.
Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: An analysis from the JUPITER trial. Lancet. 2012;380(9841):565–71.
Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: A meta-analysis. JAMA. 2011;305(24):2556–64.
Hashemi M, Hoshyar R, Ande SR, et al. Mevalonate cascade and its regulation in cholesterol metabolism in different tissues in health and disease. Curr Mol Pharmacol. 2017;10(1):13–26.
Miziorko HM. Enzymes of the mevalonate pathway of isoprenoid biosynthesis. Arch Biochem Biophys. 2011;505(2):131–43.
Wang M, Casey PJ. Protein prenylation: Unique fats make their mark on biology. Nat Rev Mol Cell Biol. 2016;17(2):110–22.
Kelly P, Bailey CL, Fueger PT, Newgard CB, Casey PJ, Kimple ME. Rap1 promotes multiple pancreatic islet cell functions and signals through mammalian target of rapamycin complex 1 to enhance proliferation. J Biol Chem. 2010;285(21):15777–85.
Takahashi H, Shibasaki T, Park JH, et al. Role of Epac2A/Rap1 signaling in interplay between incretin and sulfonylurea in insulin secretion. Diabetes. 2015;64(4):1262–72.
Veluthakal R, Thurmond DC. Emerging roles of small GTPases in islet beta-cell function. Cells. 2021;10(6).
Kaneko K, Xu P, Cordonier EL, et al. Neuronal Rap1 regulates energy balance, glucose homeostasis, and leptin actions. Cell Rep. 2016;16(11):3003–15.
•• Kaneko K, Lin HY, Fu Y, et al. Rap1 in the VMH regulates glucose homeostasis. JCI Insight. 2021;6(11). This article showed that Rap1 inhibition in VMH lower glucose levels without a change in body weight.
Shimazu T, Fukuda A, Ban T. Reciprocal influences of the ventromedial and lateral hypothalamic nuclei on blood glucose level and liver glycogen content. Nature. 1966;210(5041):1178–9.
Orekhov AN. LDL and foam cell formation as the basis of atherogenesis. Curr Opin Lipidol. 2018;29(4):279–84.
Tabas I. 2016 Russell Ross memorial lecture in vascular biology: Molecular-cellular mechanisms in the progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2017;37(2):183–9.
Alexander Y, Osto E, Schmidt-Trucksass A, et al. Endothelial function in cardiovascular medicine: A consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc Res. 2021;117(1):29–42.
Lakshmikanthan S, Zheng X, Nishijima Y, et al. Rap1 promotes endothelial mechanosensing complex formation, NO release and normal endothelial function. EMBO Rep. 2015;16(5):628–37.
Lakshmikanthan S, Sobczak M, Li Calzi S, Shaw L, Grant MB, Chrzanowska-Wodnicka M. Rap1B promotes VEGF-induced endothelial permeability and is required for dynamic regulation of the endothelial barrier. J Cell Sci. 2018;131(1).
Birukova AA, Meng F, Tian Y, et al. Prostacyclin post-treatment improves LPS-induced acute lung injury and endothelial barrier recovery via Rap1. Biochim Biophys Acta. 2015;1852(5):778–91.
•• Singh B, Kosuru R, Lakshmikanthan S, et al. Endothelial Rap1 (Ras-association proximate 1) restricts inflammatory signaling to protect from the progression of atherosclerosis. Arterioscler Thromb Vasc Biol 2021, 41(2):638–650. This study revealed that Rap1 activation in endothelial cells is protective against atherosclerotic lesion formation in mice.
Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: A dynamic balance. Nat Rev Immunol. 2013;13(10):709–21.
Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61(4):404–10.
Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31.
Tang S, Chen T, Yu Z, et al. RasGRP3 limits Toll-like receptor-triggered inflammatory response in macrophages by activating Rap1 small GTPase. Nat Commun. 2014;5:4657.
Wu H, Li Z, Yang Y, et al. Rap1A accelerates homocysteine-induced ANA-1 cells inflammation via synergy of FoxO1 and DNMT3a. Cell Signal. 2023;106:110627.
Zhang D, Jiang X, Fang P, et al. Hyperhomocysteinemia promotes inflammatory monocyte generation and accelerates atherosclerosis in transgenic cystathionine beta-synthase-deficient mice. Circulation. 2009;120(19):1893–902.
Basatemur GL, Jorgensen HF, Clarke MCH, Bennett MR, Mallat Z. Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol. 2019;16(12):727–44.
Li Q, Teng Y, Wang J, Yu M, Li Y, Zheng H. Rap1 promotes proliferation and migration of vascular smooth muscle cell via the ERK pathway. Pathol Res Pract. 2018;214(7):1045–50.
Coly PM, Boulanger CM. Role of extracellular vesicles in atherosclerosis: An update. J Leukoc Biol. 2022;111(1):51–62.
Perdomo L, Vidal-Gomez X, Soleti R, et al. Large extracellular vesicle-associated Rap1 accumulates in atherosclerotic plaques, correlates with vascular risks and is involved in atherosclerosis. Circ Res. 2020;127(6):747–60.
Funding
This work was supported by an American Heart Association Transformational Project Award (971273) to L.O. and American Heart Association Research Supplement to Promote Diversity in Science (23DIVSUP1071039) to B.T.
Author information
Authors and Affiliations
Contributions
H.A., B.T., A.K.S. and L.O. wrote the manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Agarwal, H., Tinsley, B., Sarecha, A.K. et al. Rap1 in the Context of PCSK9, Atherosclerosis, and Diabetes. Curr Atheroscler Rep 25, 931–937 (2023). https://doi.org/10.1007/s11883-023-01162-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-023-01162-7