Abstract
Purpose of Review
To discuss advances on the RNA-targeted therapies to treat dyslipidemia with the aim of reducing atherosclerotic cardiovascular disease (ASCVD).
Recent Findings
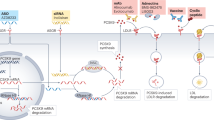
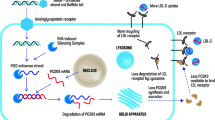
Genetic studies have paved the way for therapies that reduce translation of proteins that play causal roles in dyslipidemia and atherosclerosis like proprotein convertase subtilisin/kexin type 9 (PCSK9), apolipoprotein B-100 (apoB), apolipoprotein(a) [apo(a)], apolipoprotein C3 (apoC3), and angiopoietin-like 3 (ANGPTL3). Either antisense oligonucleotide (ASO) therapies and small interfering RNA (siRNA) molecules inhibit protein synthesis and consequently improve dyslipidemia. Most of these molecules contain N-acetylgalactosamine (GalNAc) moieties that have high specificity for hepatocytes and therefore reduce concentration in other tissues. Inclisiran, an siRNA for PCSK9, has shown robust LDL-C reductions, with good tolerability, in severe forms of hypercholesterolemia as well as in high cardiovascular disease patients with injections every 3 to 6 months. Pelacarsen is an ASO against apolipoprotein(a) that reduces Lp(a) up to 80% with good tolerability. Either inclisiran or pelacarsen is being tested to show it can prevent ASCVD. AMG 890, an siRNA compound aimed at reducing apo(a) synthesis, is also under investigation. Volanesorsen is an ASO against apoC3 that reduces triglyceride levels up to 70% and is being tested in severe hypertriglyceridemic patients. Vupanorsen is an ASO against ANGPTL3 that reduced triglyceride levels 36–53% among moderate hypertriglyceridemic individuals. Interestingly, it also reduces ApoC3 and non-HDL cholesterol and apoB; however, it lowers HDL cholesterol.
Summary
RNA-targeted therapies are being extensively tested for dyslipidemia treatment with promising results.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Roth GA, Forouzanfar MH, Moran AE, Barber R, Nguyen G, Feigin VL, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. 2015;372(14):1333–41. https://doi.org/10.1056/NEJMoa1406656.
Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–72. https://doi.org/10.1093/eurheartj/ehx144.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2018;73:e285–350. https://doi.org/10.1016/j.jacc.2018.11.003.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–88. https://doi.org/10.1093/eurheartj/ehz455.
Cholesterol Treatment Trialists C, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–81. https://doi.org/10.1016/S0140-6736(10)61350-5.
Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388(10059):2532–61. https://doi.org/10.1016/S0140-6736(16)31357-5.
Danchin N, Almahmeed W, Al-Rasadi K, Azuri J, Berrah A, Cuneo CA, et al. Achievement of low-density lipoprotein cholesterol goals in 18 countries outside Western Europe: The International ChoLesterol management Practice Study (ICLPS). Eur J Prev Cardiol. 2018;25(10):1087–94. https://doi.org/10.1177/2047487318777079.
Ray KK. Changing the paradigm for post-MI cholesterol lowering from intensive statin monotherapy towards intensive lipid-lowering regimens and individualized care. Eur Heart J. 2021;42(3):253–6. https://doi.org/10.1093/eurheartj/ehaa1008.
Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–97. https://doi.org/10.1056/NEJMoa1410489.
Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–22. https://doi.org/10.1056/NEJMoa1615664.
Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and Cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–107. https://doi.org/10.1056/NEJMoa1801174.
Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. Jama. 2007;298(3):299–308. https://doi.org/10.1001/jama.298.3.299.
Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. Jama. 2007;298(3):309–16. https://doi.org/10.1001/jama.298.3.309.
Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384(9943):626–35. https://doi.org/10.1016/S0140-6736(14)61177-6.
Cao YX, Zhang HW, Jin JL, Liu HH, Zhang Y, Xu RX, et al. Prognostic utility of triglyceride-rich lipoprotein-related markers in patients with coronary artery disease. J Lipid Res. 2020;61(9):1254–62. https://doi.org/10.1194/jlr.RA120000746.
Schwartz GG, Abt M, Bao W, DeMicco D, Kallend D, Miller M, et al. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol. 2015;65(21):2267–75. https://doi.org/10.1016/j.jacc.2015.03.544.
Macchi C, Sirtori CR, Corsini A, Santos RD, Watts GF, Ruscica M. A new dawn for managing dyslipidemias: the era of rna-based therapies. Pharmacol Res. 2019;150:104413. https://doi.org/10.1016/j.phrs.2019.104413.
Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. Jama. 2009;301(22):2331–9. https://doi.org/10.1001/jama.2009.801.
Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361(26):2518–28. https://doi.org/10.1056/NEJMoa0902604.
Burgess S, Ference BA, Staley JR, Freitag DF, Mason AM, Nielsen SF, et al. Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: a Mendelian randomization analysis. JAMA Cardiol. 2018;3(7):619–27. https://doi.org/10.1001/jamacardio.2018.1470.
Katzmann JL, Packard CJ, Chapman MJ, Katzmann I, Laufs U. Targeting RNA with antisense oligonucleotides and small interfering RNA: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76(5):563–79. https://doi.org/10.1016/j.jacc.2020.05.070.
Ito MK, Santos RD. PCSK9 inhibition with monoclonal antibodies: modern management of hypercholesterolemia. J Clin Pharmacol. 2017;57(1):7–32. https://doi.org/10.1002/jcph.766.
Bilen O, Ballantyne CM. Bempedoic acid (ETC-1002): an investigational inhibitor of ATP citrate lyase. Curr Atheroscler Rep. 2016;18(10):61. https://doi.org/10.1007/s11883-016-0611-4.
•• Rosenson RS, Burgess LJ, Ebenbichler CF, Baum SJ, ESG S, Ali S, et al. Evinacumab in patients with refractory hypercholesterolemia. N Engl J Med. 2020;383(24):2307–19. https://doi.org/10.1056/NEJMoa2031049Evinacumab, a monoclonal antibody against ANGPTL3, reduced LDL-C by 50% in hypercholesterolemic patients that were refractory to statins and PCSK9 inhibitors. This study tested either intravenous and subcutaneous injections of evinacumab.
Ray KK, Landmesser U, Leiter LA, Kallend D, Dufour R, Karakas M, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376(15):1430–40. https://doi.org/10.1056/NEJMoa1615758.
•• Witztum JL, Gaudet D, Freedman SD, Alexander VJ, Digenio A, Williams KR, et al. Volanesorsen and triglyceride levels in familial chylomicronemia syndrome. N Engl J Med. 2019;381(6):531–42. https://doi.org/10.1056/NEJMoa1715944In the APPROACH study, volanesorsen, a second-generation ASO against ApoC3, reduced triglycerides by 70% in comparison with placebo in patients with familial chylomicronemia syndrom (FCS). The use of volanesorsen was associated with reduction in episodes of acute pancreatitis when compared with the incidence occuring before the study. This non-GalNAc conjugated formulation was associated with injection site reactions and reduction in platelet counts.
•• Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif JC, Baum SJ. Steinhagen-Thiessen E et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med. 2020;382(3):244–55. https://doi.org/10.1056/NEJMoa1905239On this double-blinded randomized-controlled trial, the ASO against apolipoprotein(a) pelacarsen was tested in different subcutaneous doses of 20, 40, or 60 mg every 4 weeks; 20 mg every 2 weeks; or 20 mg every week in patients with previous CVD and elevated Lp(a) for 6 to 12 months. There was dose-dependent reduction in Lp(a) with mean percent decreases of 35% at a dose of 20 mg every 4 weeks, 56% at 40 mg every 4 weeks, 58% at 20 mg every 2 weeks, 72% at 60 mg every 4 weeks, and 80% at 20 mg every week, as compared with 6% with placebo. The reduction in oxydized phospholipids, pro-inflammatory compounds carried by Lp(a) particles, was also proportional to the dosage. No differences were seen regarding adverse events in comparison with placebo; however, as expected, the most frequent adverse events were injection site reactions 27% in those receving pelacarsen vs. 6% in the placebo group.
•• Gaudet D, Karwatowska-Prokopczuk E, Baum SJ, Hurh E, Kingsbury J, Bartlett VJ, et al. Vupanorsen, an N-acetyl galactosamine-conjugated antisense drug to ANGPTL3 mRNA, lowers triglycerides and atherogenic lipoproteins in patients with diabetes, hepatic steatosis, and hypertriglyceridaemia. Eur Heart J. 2020;41(40):3936–45. https://doi.org/10.1093/eurheartj/ehaa689On this double-blind randommized clinical trial, the ASO against ANGPTL3 vupanorsen was tested in patients with diabetes, moderate hypetriglyceridemia, and hepatic steatosis. Overall, there was a 44% reduction in triglycerides, the main study endpoint. Of importance, vupanorsen also reduced ApoC3 by 58%, and this opens the possibility of prevention of cardiovascular events since ApoC3 exerts a series of pro-atherogenic mechanisms. As shown in genetic studies with loss-of-function variants of ANGPTL3, vupanorsen also reduced HDL-C by 24%; if this has clinical significance needs to be determined. There was no effect on glucose control and redcution in steatosis. Fruther studies are guaranteed with ANGPTL3 inhibitors.
Nordestgaard BG, Nicholls SJ, Langsted A, Ray KK, Tybjaerg-Hansen A. Advances in lipid-lowering therapy through gene-silencing technologies. Nat Rev Cardiol. 2018;15(5):261–72. https://doi.org/10.1038/nrcardio.2018.3.
Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354(12):1264–72. https://doi.org/10.1056/NEJMoa054013.
•• Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382(16):1507–19. https://doi.org/10.1056/NEJMoa1912387This randomized controlled study shows LDL-C reductions of about 50% induced by 300 mg inclisiran injections every 6 months in patients at high risk of cardiovascular disease that persisted with elevated LDL-C despite use of statins and or ezetimibe. Inclsiran showed good tolerability, with injection site reactions being the most frequent adverse events.
•• Raal FJ, Kallend D, Ray KK, Turner T, Koenig W, Wright RS, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020;382(16):1520–30. https://doi.org/10.1056/NEJMoa1913805This randomized double-blind placebo-controlled study showed that inclisiran 300 mg inejected at days 1, 90, 270, and 450 reduced LDL-C by − 47.9% (95% CI, − 53.5 to − 42.3; P < 0.001), time averaged reductions at days 90 and 540, in comparison with placebo in heterozygous familial hypercholesterolemia patients that were in use of standard lipid-lowering therapy and baseline LDL-C of approximately 153 mg/dL. LDL-C was reduced independently of FH genotype. There were no differences in adverse events in comparison with placebo. Anti-drug antibodies were detected in low titers in 2.6% of study subjects, were transient, and were not associated with reduction in inclisiran efficacy.
Lamina C, Kronenberg F, Lp GC. Estimation of the required lipoprotein(a)-lowering therapeutic effect size for reduction in coronary heart disease outcomes: a Mendelian randomization analysis. JAMA Cardiol. 2019;4:575–9. https://doi.org/10.1001/jamacardio.2019.1041.
Tg HW. Group of the Exome Sequencing Project NHL, Blood I, Crosby J, Peloso GM, Auer PL et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371(1):22–31. https://doi.org/10.1056/NEJMoa1307095.
Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371(1):32–41. https://doi.org/10.1056/NEJMoa1308027.
Stitziel NO, Khera AV, Wang X, Bierhals AJ, Vourakis AC, Sperry AE, et al. ANGPTL3 deficiency and protection against coronary artery disease. J Am Coll Cardiol. 2017;69(16):2054–63. https://doi.org/10.1016/j.jacc.2017.02.030.
Dewey FE, Gusarova V, Dunbar RL, O'Dushlaine C, Schurmann C, Gottesman O, et al. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med. 2017;377(3):211–21. https://doi.org/10.1056/NEJMoa1612790.
Graham MJ, Lee RG, Brandt TA, Tai LJ, Fu W, Peralta R, et al. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N Engl J Med. 2017;377(3):222–32. https://doi.org/10.1056/NEJMoa1701329.
Santos RD, Duell PB, East C, Guyton JR, Moriarty PM, Chin W, et al. Long-term efficacy and safety of mipomersen in patients with familial hypercholesterolaemia: 2-year interim results of an open-label extension. Eur Heart J. 2015;36(9):566–75. https://doi.org/10.1093/eurheartj/eht549.
• Blom DJ, Raal FJ, Santos RD, Marais AD. Lomitapide and mipomersen-inhibiting microsomal triglyceride transfer protein (MTP) and apoB100 synthesis. Curr Atheroscler Rep. 2019;21(12):48. https://doi.org/10.1007/s11883-019-0809-3Review on the ASO for apolipoprotein B mipomersen, a non GalNAc conjugated molecule, focusing on its effcacy, LDL-C lowering by 25%, and adverse events, mostly injection site reactions and flu-like symptoms, liver fat accumulation was intrisic to reduction in apoB production.
Khvorova A. Oligonucleotide therapeutics - a new class of cholesterol-lowering drugs. N Engl J Med. 2017;376(1):4–7. https://doi.org/10.1056/NEJMp1614154.
Ragusa R, Basta G, Neglia D, De Caterina R, Del Turco S, Caselli C. PCSK9 and atherosclerosis: looking beyond LDL regulation. Eur J Clin Investig. 2020:e13459. https://doi.org/10.1111/eci.13459.
Stoekenbroek RM, Lambert G, Cariou B, Hovingh GK. Inhibiting PCSK9 - biology beyond LDL control. Nat Rev Endocrinol. 2018;15(1):52–62. https://doi.org/10.1038/s41574-018-0110-5.
Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34(2):154–6. https://doi.org/10.1038/ng1161.
Seidah NG, Prat A, Pirillo A, Catapano AL, Norata GD. Novel strategies to target proprotein convertase subtilisin kexin 9: beyond monoclonal antibodies. Cardiovasc Res. 2019;115(3):510–8. https://doi.org/10.1093/cvr/cvz003.
Brandts J, Ray KK. Small interfering RNA to proprotein convertase subtilisin/kexin type 9: transforming LDL-cholesterol-lowering strategies. Curr Opin Lipidol. 2020;31(4):182–6. https://doi.org/10.1097/MOL.0000000000000691.
Schmidt AF, Pearce LS, Wilkins JT, Overington JP, Hingorani AD, Casas JP. PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2017;4:CD011748. https://doi.org/10.1002/14651858.CD011748.pub2.
Fitzgerald K, White S, Borodovsky A, Bettencourt BR, Strahs A, Clausen V, et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med. 2017;376(1):41–51. https://doi.org/10.1056/NEJMoa1609243.
Khan SA, Naz A, Qamar Masood M, Shah R. Meta-analysis of inclisiran for the treatment of hypercholesterolemia. Am J Cardiol. 2020;134:69–73. https://doi.org/10.1016/j.amjcard.2020.08.018.
Giugliano RP, Mach F, Zavitz K, Kurtz C, Im K, Kanevsky E, et al. Cognitive function in a randomized trial of evolocumab. N Engl J Med. 2017;377(7):633–43. https://doi.org/10.1056/NEJMoa1701131.
Banerjee Y, Santos RD, Al-Rasadi K, Rizzo M. Targeting PCSK9 for therapeutic gains: have we addressed all the concerns? Atherosclerosis. 2016;248:62–75. https://doi.org/10.1016/j.atherosclerosis.2016.02.018.
Landmesser U, Haghikia A, Leiter LA, Wright RS, Kallend D, Wijngaard P, et al. Effect of inclisiran, the small-interfering RNA against proprotein convertase subtilisin/kexin type 9, on platelets, immune cells, and immunological biomarkers: a pre-specified analysis from ORION-1. Cardiovasc Res. 2021;117(1):284–91. https://doi.org/10.1093/cvr/cvaa077.
Kosmas CE, Munoz Estrella A, Skavdis A, Pena Genao E, Martinez I, Guzman E. Inclisiran for the treatment of cardiovascular disease: a short review on the emerging data and therapeutic potential. Ther Clin Risk Manag. 2020;16:1031–7. https://doi.org/10.2147/TCRM.S230592.
Kamstrup PR. Lipoprotein(a) and cardiovascular disease. Clin Chem. 2021;67(1):154–66. https://doi.org/10.1093/clinchem/hvaa247.
Hegele RA, Tsimikas S. Lipid-lowering agents. Circ Res. 2019;124(3):386–404. https://doi.org/10.1161/CIRCRESAHA.118.313171.
Emerging Risk Factors C, Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. Jama. 2009;302(4):412–23. https://doi.org/10.1001/jama.2009.1063.
O'Donoghue ML, Fazio S, Giugliano RP, Stroes ESG, Kanevsky E, Gouni-Berthold I, et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation. 2019;139(12):1483–92. https://doi.org/10.1161/circulationaha.118.037184.
Bittner VA, Szarek M, Aylward PE, Bhatt DL, Diaz R, Edelberg JM, et al. Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol. 2020;75(2):133–44. https://doi.org/10.1016/j.jacc.2019.10.057.
Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63(5):470–7. https://doi.org/10.1016/j.jacc.2013.09.038.
Tsimikas S, Viney NJ, Hughes SG, Singleton W, Graham MJ, Baker BF, et al. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet. 2015;386(10002):1472–83. https://doi.org/10.1016/S0140-6736(15)61252-1.
Viney NJ, van Capelleveen JC, Geary RS, Xia S, Tami JA, Yu RZ, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388(10057):2239–53. https://doi.org/10.1016/S0140-6736(16)31009-1.
Tsimikas S, Gordts P, Nora C, Yeang C, Witztum JL. Statin therapy increases lipoprotein(a) levels. Eur Heart J. 2020;41(24):2275–84. https://doi.org/10.1093/eurheartj/ehz310.
van Dijk KW, Rensen PC, Voshol PJ, Havekes LM. The role and mode of action of apolipoproteins CIII and AV: synergistic actors in triglyceride metabolism? Curr Opin Lipidol. 2004;15(3):239–46. https://doi.org/10.1097/00041433-200406000-00002.
Hussain A, Ballantyne CM, Saeed A, Virani SS. Triglycerides and ASCVD risk reduction: recent insights and future directions. Curr Atheroscler Rep. 2020;22(7):25. https://doi.org/10.1007/s11883-020-00846-8.
Windler E, Havel RJ. Inhibitory effects of C apolipoproteins from rats and humans on the uptake of triglyceride-rich lipoproteins and their remnants by the perfused rat liver. J Lipid Res. 1985;26(5):556–65.
Mendivil CO, Zheng C, Furtado J, Lel J, Sacks FM. Metabolism of very-low-density lipoprotein and low-density lipoprotein containing apolipoprotein C-III and not other small apolipoproteins. Arterioscler Thromb Vasc Biol. 2010;30(2):239–45. https://doi.org/10.1161/ATVBAHA.109.197830.
Dallinga-Thie GM, Kroon J, Boren J, Chapman MJ. Triglyceride-rich lipoproteins and remnants: targets for therapy? Curr Cardiol Rep. 2016;18(7):67. https://doi.org/10.1007/s11886-016-0745-6.
Wyler von Ballmoos MC, Haring B, Sacks FM. The risk of cardiovascular events with increased apolipoprotein CIII: a systematic review and meta-analysis. J Clin Lipidol. 2015;9(4):498–510. https://doi.org/10.1016/j.jacl.2015.05.002.
Gaudet D, Alexander VJ, Baker BF, Brisson D, Tremblay K, Singleton W, et al. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N Engl J Med. 2015;373(5):438–47. https://doi.org/10.1056/NEJMoa1400283.
Gouni-Berthold I, Alexander V, Digenio A, DuFour R, Steinhagen-Thiessen E, Martin S, et al. Apolipoprotein C-III inhibition with volanesorsen in patients with hypertriglyceridemia (COMPASS): a randomized, double-blind, placebo-controlled trial. Atheroscler Suppl. 2017;28:E1–2. https://doi.org/10.1016/j.atherosclerosissup.2017.08.003.
Esan O, Wierzbicki AS. Volanesorsen in the treatment of familial chylomicronemia syndrome or hypertriglyceridaemia: design, development and place in therapy. Drug Des Devel Ther. 2020;14:2623–36. https://doi.org/10.2147/DDDT.S224771.
Paik J, Duggan S. Volanesorsen: first global approval. Drugs. 2019;79(12):1349–54. https://doi.org/10.1007/s40265-019-01168-z.
Kersten S. Angiopoietin-like 3 in lipoprotein metabolism. Nat Rev Endocrinol. 2017;13(12):731–9. https://doi.org/10.1038/nrendo.2017.119.
•• Raal FJ, Rosenson RS, Reeskamp LF, Hovingh GK, JJP K, Rubba P, et al. Evinacumab for homozygous familial hypercholesterolemia. N Engl J Med. 2020;383(8):711–20. https://doi.org/10.1056/NEJMoa2004215Intravenous injections of 15 mg/kg of evinacumab, a monoclonal antibody against ANGPTL3, reduced LDL-C by 49% (95% CI, − 65.0 to − 33.1; P < 0.001) in comparison with placebo in homozygous familial hypercholesterolemia patients (N = 65 randomized 2:1 to evinacumab). Reductions in LDL-C occurred independently of the genotype and type of LDL receptor defect. This seminal study suggests that inhibition of ANGPTL3 will be a revolutionary treatment for this disease.
Funding
RDS is the recipient of a scholarship from Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico, Brazil, (CNPq) #303734/2018-3.
Author information
Authors and Affiliations
Contributions
MHM, VZR, and RDS have reviewed the literature and drafted the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
MHM has received honoraria related to speaker activities from Amgen and EMS.
VZR has received honoraria related to speaker activities from Ache, Amgen, Novo Nordisk, and Sanofi.
RDS has received honoraria related to consulting, research, and/or speaker activities from Abbott, Ache, Amgen, AstraZeneca, Esperion, EMS, Getz Pharma, Kowa, Libbs, Novo Nordisk, Novartis, Merck, MSD, Pfizer, PTC Therapeutics, and Sanofi.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Coronary Heart Disease
Rights and permissions
About this article
Cite this article
Miname, M.H., Rocha, V.Z. & Santos, R.D. The Role of RNA-Targeted Therapeutics to Reduce ASCVD Risk: What Have We Learned Recently?. Curr Atheroscler Rep 23, 40 (2021). https://doi.org/10.1007/s11883-021-00936-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s11883-021-00936-1