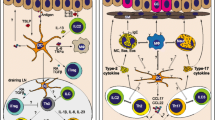
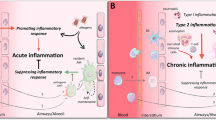
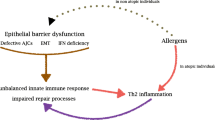
Abstract
Promoting tolerance to inhaled antigens is an active area of study with the potential to benefit the millions of Americans currently suffering from respiratory allergies and asthma. Interestingly, not all individuals with atopy are symptomatic, arguing that sensitization alone does not lead to an allergic clinical phenotype. Respiratory dendritic cells (rDCs), classically associated with inducing inflammatory responses, can actively promote tolerance. Tolerance can be broken when inflammatory stimuli, including viral infections and other environmental exposures, inhibit rDC-mediated tolerance by allowing innocuous antigen to be presented to initiate type-2 immunity. Importantly, rDCs are composed of multiple subsets, each with a unique response to an inhaled antigen that can lead to either tolerance or inflammation. In this review, we will discuss how rDC subsets actively maintain tolerance or, alternatively, break tolerance in response to environmental cues.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hoppin JA, Jaramillo R, Salo P, Sandler DP, London SJ, Zeldin DC. Questionnaire predictors of atopy in a US population sample: findings from the National Health and Nutrition Examination Survey. Am J Epidemiol. 2011;173(5):544–52.
Genuneit J, Strachan DP, Buchele G, Weber J, Loss G, Sozanska B, et al. The combined effects of family size and farm exposure on childhood hay fever and atopy. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2013;24(3):293–8.
Schram-Bijkerk D, Doekes G, Douwes J, Boeve M, Riedler J, Ublagger E, et al. Bacterial and fungal agents in house dust and wheeze in children: the PARSIFAL study. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2005;35(10):1272–8.
Loss G, Apprich S, Waser M, Kneifel W, Genuneit J, Buchele G, et al. The protective effect of farm milk consumption on childhood asthma and atopy: the GABRIELA study. The Journal of Allergy and Clinical Immunology. 2011;128(4):766–73.e4.
Behbod B, Sordillo JE, Hoffman EB, Datta S, Webb TE, Kwan DL, Kamel JA, Muilenberg ML, Scott JA, Chew GL, et al. Asthma & allergy development: contrasting influences of yeasts & other fungal exposures. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2014.
Karvonen AM, Hyvarinen A, Rintala H, Korppi M, Taubel M, Doekes G, et al. Quantity and diversity of environmental microbial exposure and development of asthma: a birth cohort study. Allergy. 2014;69(8):1092–101.
Ege MJ, Frei R, Bieli C, Schram-Bijkerk D, Waser M, Benz MR, et al. Not all farming environments protect against the development of asthma and wheeze in children. The Journal of Allergy and Cinical Immunology. 2007;119(5):1140–7.
Backman K, Piippo-Savolainen E, Ollikainen H, Koskela H, and Korppi M. Adults face increased asthma risk after infant RSV bronchiolitis and reduced respiratory health-related quality of life after RSV pneumonia. Acta paediatrica (Oslo, Norway : 1992). 2014.
Caliskan M, Bochkov YA, Kreiner-Moller E, Bonnelykke K, Stein MM, Du G, et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368(15):1398–407.
Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178(7):667–72.
Maheswaran D, Zeng Y, Chan-Yeung M, Scott J, Osornio-Vargas A, Becker AB, et al. Exposure to Beta-(1,3)-d-glucan in house dust at age 7–10 is associated with airway hyperresponsiveness and atopic asthma by age 11–14. PLoS One. 2014;9(6):e98878.
Matsuse H, Tsuchida T, Fukahori S, Kawano T, Tomari S, Matsuo N, et al. Differential airway inflammatory responses in asthma exacerbations induced by respiratory syncytial virus and influenza virus a. Int Arch Allergy Immunol. 2013;161(4):378–82.
Stelmaszczyk-Emmel A, Zawadzka-Krajewska A, Szypowska A, Kulus M, Demkow U. Frequency and activation of CD4+CD25 FoxP3+ regulatory T cells in peripheral blood from children with atopic allergy. Int Arch Allergy Immunol. 2013;162(1):16–24.
Lluis A, Depner M, Gaugler B, Saas P, Casaca VI, Raedler D, et al. Increased regulatory T-cell numbers are associated with farm milk exposure and lower atopic sensitization and asthma in childhood. The Journal of Allergy and cCinical Immunology. 2014;133(2):551–9.
Boudousquie C, Pellaton C, Barbier N, Spertini F. CD4+CD25+ T cell depletion impairs tolerance induction in a murine model of asthma. Clinical and Experimental Allergy : Journal of the British Society for Allergy and Clinical Immunology. 2009;39(9):1415–26.
Raitala A, Karjalainen J, Oja SS, Kosunen TU, Hurme M. Indoleamine 2,3-dioxygenase (IDO) activity is lower in atopic than in non-atopic individuals and is enhanced by environmental factors protecting from atopy. Mol Immunol. 2006;43(7):1054–6.
Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. Journal of Immunology. 2009;83(4):2475–83.
Yan Y, Zhang GX, Gran B, Fallarino F, Yu S, Li H, et al. DO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. Journal of Immunolog. 2010;185(10):5953–61.
van der Sluijs KF, van de Pol MA, Kulik W, Dijkhuis A, Smids BS, van Eijk HW, et al. Systemic tryptophan and kynurenine catabolite levels relate to severity of rhinovirus-induced asthma exacerbation: a prospective study with a parallel-group design. Thorax. 2013;68(12):1122–30.
Brehm JM, Acosta-Perez E, Klei L, Roeder K, Barmada M, Boutaoui N, et al. Vitamin D insufficiency and severe asthma exacerbations in Puerto Rican children. Am J Respir Crit Care Med. 2012;186(2):140–6.
Checkley W, Robinson CL, Baumann LM, Hansel NN, Romero K, Pollard SL, Wise RA, Gilman RH, Mougey E, and Lima JJ. 25-hydroxy vitamin D levels are associated with childhood asthma in a population-based study in Peru. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2014.
Thuesen BH, Skaaby T, Husemoen LL, Fenger M, Jorgensen T, and Linneberg A. The association of serum 25-OH vitamin D with atopy, asthma, and lung function in a prospective study of Danish adults. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2014.
Yao TC, Tu YL, Chang SW, Tsai HJ, Gu PW, Ning HC, et al. Suboptimal vitamin D status in a population-based study of Asian children: prevalence and relation to allergic diseases and atopy. PLoS One. 2014;9(6):e99105.
von Bubnoff D, Fimmers R, Bogdanow M, Matz H, Koch S, Bieber T. Asymptomatic atopy is associated with increased indoleamine 2,3-dioxygenase activity and interleukin-10 production during seasonal allergen exposure. Clinical and Experimental Allergy : Journal of the British Society for Allergy and Clinical Immunology. 2004;34(7):1056–63.
Starkhammar M, Larsson O, Kumlien Georen S, Leino M, Dahlen SE, Adner M, et al. Toll-like receptor ligands LPS and poly (I:C) exacerbate airway hyperresponsiveness in a model of airway allergy in mice, independently of inflammation. PLoS One. 2014;9(8):e104114.
Delayre-Orthez C, de Blay F, Frossard N, Pons F. Dose-dependent effects of endotoxins on allergen sensitization and challenge in the mouse. Clinical and Experimental Allergy : Journal of the British Society for Allergy and Clinical Immunology. 2004;34(11):1789–95.
Lam D, Ng N, Lee S, Batzer G, Horner AA. Airway house dust extract exposures modify allergen-induced airway hypersensitivity responses by TLR4-dependent and independent pathways. Journal of immunology. 2008;181(4):2925–32.
Ng N, Lam D, Paulus P, Batzer G, Horner AA. House dust extracts have both TH2 adjuvant and tolerogenic activities. The Journal of Allergy and Clinical Immunology. 2006;117(5):1074–81.
Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA : the journal of the American Medical Association. 2002;288(8):963–72.
Debarry J, Garn H, Hanuszkiewicz A, Dickgreber N, Blumer N, von Mutius E, et al. Acinetobacter lwoffii and Lactococcus lactis strains isolated from farm cowsheds possess strong allergy-protective properties. The Journal of Allergy and cCinical Immunology. 2007;119(6):1514–21.
Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30(1):16–34.
van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201(6):981–91.
Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–61.
Mazzini E, Massimiliano L, Penna G, Rescigno M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1(+) macrophages to CD103(+) dendritic cells. Immunity. 2014;40(2):248–61.
Khandelwal P, Blanco-Mezquita T, Emami P, Lee HS, Reyes NJ, Mathew R, et al. Ocular mucosal CD11b+ and CD103+ mouse dendritic cells under normal conditions and in allergic immune responses. PLoS One. 2013;8(5):e64193.
Khare A, Krishnamoorthy N, Oriss TB, Fei M, Ray P, Ray A. Cutting edge: inhaled antigen upregulates retinaldehyde dehydrogenase in lung CD103+ but not plasmacytoid dendritic cells to induce Foxp3 de novo in CD4+ T cells and promote airway tolerance. Journal of Immunology (Baltimore, Md : 1950). 2013;191(1):25–9. The authors find that CD103+ DCs are critical for airway tolerance by using the BATF3-KO mouse that is deficient in this DC subset. The BATF3-KO mice do not develop tolerance to inhaled OVA antigen compared to that of wild-type mice and fail to induce Tregs in the lungs.
Nakano H, Free ME, Whitehead GS, Maruoka S, Wilson RH, Nakano K, et al. Pulmonary CD103(+) dendritic cells prime Th2 responses to inhaled allergens. Mucosal Immunology. 2012;5(1):53–65.
Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. The Journal of Experimental Medicine. 2010;207(6):1247–60.
Chu CC, Ali N, Karagiannis P, Di Meglio P, Skowera A, Napolitano L, et al. Resident CD141 (BDCA3)+ dendritic cells in human skin produce IL-10 and induce regulatory T cells that suppress skin inflammation. The Journal of Experimental Medicine. 2012;209(5):935–45.
Penna G, Amuchastegui S, Giarratana N, Daniel KC, Vulcano M, Sozzani S, et al. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J Immunol. 1950;178(1):145–53.
de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. The Journal of Experimental Medicine. 2004;200(1):89–98.
Lombardi V, Speak AO, Kerzerho J, Szely N, Akbari O. CD8alpha(+)beta(−) and CD8alpha(+)beta(+) plasmacytoid dendritic cells induce Foxp3(+) regulatory T cells and prevent the induction of airway hyper-reactivity. Mucosal Immunology. 2012;5(4):432–43. This study finds that multiple subpopulations of pDCs reside in the airways. The adoptive transfer of CD8alpha(+) pDCs, but not CD8alpha(−) pDCs, into mice is sufficient to induce differentiation of naïve CD4 T cells into Tregs.
Sharma MD, Hou DY, Liu Y, Koni PA, Metz R, Chandler P, et al. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009;113(24):6102–11.
Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114(2):280–90.
Ricci G, Patrizi A, Baldi E, Menna G, Tabanelli M, Masi M. Long-term follow-up of atopic dermatitis: retrospective analysis of related risk factors and association with concomitant allergic diseases. J Am Acad Dermatol. 2006;55(5):765–71.
Frieri M. Asthma linked with rhinosinusitis: an extensive review. Allergy & rhinology. 2014;5(1):41–9.
Pastorello EA, Incorvaia C, Ortolani C, Bonini S, Canonica GW, Romagnani S, et al. Studies on the relationship between the level of specific IgE antibodies and the clinical expression of allergy: I. Definition of levels distinguishing patients with symptomatic from patients with asymptomatic allergy to common aeroallergens. J Allergy Clin Immunol. 1995;96(5 Pt 1):580–7.
Manches O, Munn D, Fallahi A, Lifson J, Chaperot L, Plumas J, et al. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J Clin Invest. 2008;118(10):3431–9.
Davidson S, Kaiko G, Loh Z, Lalwani A, Zhang V, Spann K, et al. Plasmacytoid dendritic cells promote host defense against acute pneumovirus infection via the TLR7-MyD88-dependent signaling pathway. J Immunol. 2011;186(10):5938–48.
Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, et al. Inflammatory dendritic cells—not basophils—are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207(10):2097–111.
Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38(2):322–35.
Thornton EE, Looney MR, Bose O, Sen D, Sheppard D, Locksley R, et al. Spatiotemporally separated antigen uptake by alveolar dendritic cells and airway presentation to T cells in the lung. J Exp Med. 2012;209(6):1183–99.
Cook D, Burgents J, and Nakano H. Migratory properties of pulmonary dendritic cells are developmentally programmed. J Immunol. 2012;188(61).4.
Jakubzick C, Helft J, Kaplan TJ, Randolph GJ. Optimization of methods to study pulmonary dendritic cell migration reveals distinct capacities of DC subsets to acquire soluble versus particulate antigen. J Immunol Methods. 2008;337(2):121–31.
Raymond M, Rubio M, Fortin G, Shalaby KH, Hammad H, Lambrecht BN, et al. Selective control of SIRP-alpha-positive airway dendritic cell trafficking through CD47 is critical for the development of T(H)2-mediated allergic inflammation. J Allergy Clin Immunol. 2009;124(6):1333––42 e1.
Jakubzick C, Helft J, Kaplan TJ, Randolph GJ. Optimization of methods to study pulmonary dendritic cell migration reveals distinct capacities of DC subsets to acquire soluble versus particulate antigen. J Immunol Methods. 2008;337(2):121–31.
McCarthy NE, Jones HA, Marks NA, Shiner RJ, Ind PW, Al-Hassi HO, et al. Inhaled allergen-driven CD1c up-regulation and enhanced antigen uptake by activated human respiratory-tract dendritic cells in atopic asthma. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2007;37(1):72–82.
Greer AM, Matthay MA, Kukreja J, Bhakta NR, Nguyen CP, Wolters PJ, et al. Accumulation of BDCA1(+) dendritic cells in interstitial fibrotic lung diseases and Th2-high asthma. PLoS One. 2014;9(6):e99084.
Williams JW, Tjota MY, Clay BS, Vander Lugt B, Bandukwala HS, Hrusch CL, Decker DC, Blaine KM, Fixsen BR, Singh H, et al. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat Commun. 2013;4(2990)
Tjota MY, Williams JW, Lu T, Clay BS, Byrd T, Hrusch CL, et al. IL-33-dependent induction of allergic lung inflammation by FcgammaRIII signaling. J Clin Invest. 2013;123(5):2287–97.
Bandukwala HS, Clay BS, Tong J, Mody PD, Cannon JL, Shilling RA, et al. Signaling through Fc gamma RIII is required for optimal T helper type (Th)2 responses and Th2-mediated airway inflammation. J Exp Med. 2007;204(8):1875–89.
Hamerman JA, Ni M, Killebrew JR, Chu CL, Lowell CA. The expanding roles of ITAM adapters FcRgamma and DAP12 in myeloid cells. Immunol Rev. 2009;232(1):42–58.
Kitamura K, Takeda K, Koya T, Miyahara N, Kodama T, Dakhama A, et al. Critical role of the Fc receptor gamma-chain on APCs in the development of allergen-induced airway hyperresponsiveness and inflammation. J Immunol. 2007;178(1):480–8.
Barrett NA, Rahman OM, Fernandez JM, Parsons MW, Xing W, Austen KF, et al. Dectin-2 mediates Th2 immunity through the generation of cysteinyl leukotrienes. J Exp Med. 2011;208(3):593–604.
Tjota MY, Hrusch CL, Blaine KM, Williams JW, Barrett NA, and Sperling AI. Signaling through FcRgamma-associated receptors on dendritic cells drives IL-33-dependent T2-type responses. The Journal of allergy and clinical immunology. 2014. The Journal of allergy and clinical immunology. 2014. This study highlights how diverse allergens can promote Th2 responses by acting on DCs. Both a non-enzymatic allergen (OVA) and an enzymatic allergen (house dust mite) were able to utilize different DC receptors (FcRγIII or Dectin-2, respectively) to signal through FcRgamma on DCs and promote Th2 differentiation
Gao Y, Nish SA, Jiang R, Hou L, Licona-Limon P, Weinstein JS, et al. Control of T helper 2 responses by transcription factor IRF4-dependent dendritic cells. Immunity. 2013;39(4):722–32.
Kowal K, Moller HJ, Dubuske LM, Moestrup SK, Bodzenta-Lukaszyk A. Differential expression of monocyte CD163 in single- and dual-asthmatic responders during allergen-induced bronchoconstriction. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2006;36(12):1584–91.
Moniuszko M, Bodzenta-Lukaszyk A, Kowal K, Lenczewska D, Dabrowska M. Enhanced frequencies of CD14++CD16+, but not CD14+CD16+, peripheral blood monocytes in severe asthmatic patients. Clin Immunol. 2009;30(3):338–46.
Zhu XJ, Yang ZF, Chen Y, Wang J, Rosmarin AG. PU.1 is essential for CD11c expression in CD8(+)/CD8(−) lymphoid and monocyte-derived dendritic cells during GM-CSF or FLT3L-induced differentiation. PLoS One. 2012;7(12):e52141.
Bieber T. The pro- and anti-inflammatory properties of human antigen-presenting cells expressing the high affinity receptor for IgE (Fc epsilon RI). Immunobiology. 2007;212(6):499–503.
Yang IA, Fong KM, Holgate ST, Holloway JW. The role of toll-like receptors and related receptors of the innate immune system in asthma. Curr Opin Allergy Clin Immunol. 2006;6(1):23–8.
Smit LA, Siroux V, Bouzigon E, Oryszczyn MP, Lathrop M, Demenais F, et al. CD14 and toll-like receptor gene polymorphisms, country living, and asthma in adults. Am J Respir Crit Care Med. 2009;179(5):363–8.
Kucuksezer UC, Palomares O, Ruckert B, Jartti T, Puhakka T, Nandy A, et al. Triggering of specific Toll-like receptors and proinflammatory cytokines breaks allergen-specific T-cell tolerance in human tonsils and peripheral blood. The Journal of allergy and clinical immunology. 2013;131(3):875–85. This study found that human tonsils are a source of tolerogenic T cells. TLR4 or TLR8 agonists can act on myeloid DCs in the tonsil to inhibit the function of the tolerogenic T cells and induce T cell proliferation in response to allergen.
Spergel JM, Paller AS. Atopic dermatitis and the atopic march. The Journal of allergy and clinical immunology. 2003;112(6 Suppl):S118–27.
Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. The Journal of Experimental Medicine. 2005;202(9):1213–23.
Froidure A, Shen C, Gras D, Van Snick J, Chanez P, Pilette C. Myeloid dendritic cells are primed in allergic asthma for thymic stromal lymphopoietin-mediated induction of Th2 and Th9 responses. Allergy. 2014;69(8):1068–76. Patients with allergic asthma have increased expression of TSLPR on circulating myeloid DCs. These TSLPR-expressing DCs were more potent at inducing Th2 and Th9 responses when compared to DCs isolated from non-allergic patients. This suggests a global dysregulation of DCs in allergic patients that may increase susceptibility to allergen sensitization.
Ho AW, Prabhu N, Betts RJ, Ge MQ, Dai X, Hutchinson PE, et al. Lung CD103+ dendritic cells efficiently transport influenza virus to the lymph node and load viral antigen onto MHC class I for presentation to CD8 T cells. J Immunol. 2011;187(11):6011–21.
Acknowledgment
This work was supported by NIH R01HL118758, NIH 5T32HL007605 (C.L.H.), NIH U19 AI095230, and a Naomi Ragins-Goldsmith Fellowship, University of Chicago (to M.Y.T.).
Compliance with Ethics Guidelines
ᅟ
Conflict of interest
Melissa Y. Tjota and Anne I. Sperling declare that they have no conflict of interest.
Cara L. Hrusch reports grants from the NIH, during the conduct of the study.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Basic and Applied Science
Rights and permissions
About this article
Cite this article
Hrusch, C.L., Tjota, M.Y. & Sperling, A.I. The Role of Dendritic Cells and Monocytes in the Maintenance and Loss of Respiratory Tolerance. Curr Allergy Asthma Rep 15, 494 (2015). https://doi.org/10.1007/s11882-014-0494-9
Published:
DOI: https://doi.org/10.1007/s11882-014-0494-9