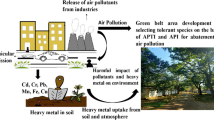
Abstract
Plants are especially useful as biological indicators to assess air pollution and the effects of urbanisation. The aim of this study was to investigate tree species’ sensitivity to air pollution using the Air Pollution Tolerance Index (APTI), the dust content (PM10) of leaves, and the heavy metal concentration of leaves. Sampling sites were in a city park (urban area), a location on the edge of the city (suburban area), and a forested location (rural area) along an urbanisation gradient in the city of Vienna in Austria. Leaf samples were collected from Acer platanoides, Fraxinus excelsior, and Quercus robur tree species. The APTI was determined by measuring the content of relative water ascorbic acid and chlorophyll, and the pH of leaf extracts. We found significant difference among species in their relative water content, pH, and content of ascorbic acid and chlorophyll Additionally, we found significant differences in chlorophyll content and pH based on sampling site. The highest chlorophyll content was found in A. platanoides. There was a significant difference among species in Al, Ba, Cr, Ni, and Zn concentrations in each stage of urbanisation. Based on APTI values, the studied species were sensitive indicators of air pollution; thus, they are useful bioindicator species, and they are suitable for air pollution monitoring. We demonstrated the effects of urbanisation with these bioindicator species with elevated PM10, Al, Ba, Cu, Fe, Sr, and Zn concentrations in urban area samples.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Air pollution is closely linked to urbanisation (Larkin et al. 2016; Hueglin et al. 2005). Wang et al. (2017) reported that urban air pollution is a critical environmental problem. Plants are valuable biological indicators to assess air pollution, due to their wide distribution (Kardel et al. 2010). They are sedentary organisms, which makes them especially suitable as integrative bioindicators of pollution (Kardel et al. 2010). Several plant species are used as bioindicators (Simon et al. 2011, 2016, 2021; Molnár et al. 2020). Air pollutants such as metals and other inorganic contaminants are emitted into the environment by transportation, industry, and fossil fuel usage (Çelik et al. 2005). Various airborne particles, such as trace elements, pollens, spores and salts, are trapped by the leaf’s surface. Based on physiological reactions, some species are good accumulators, while others are good indicators of atmospheric contamination (Simon et al. 2021). Plants near a pollution source can be screened for their sensitivity and tolerance levels for pollutants through the measurement of certain biochemical parameters, including total chlorophyll, ascorbic acid, pH of leaf extract, and relative water content. These parameters are encapsulated in a single formula called the air pollution tolerance index (APTI) (Simon et al. 2014; Kuddus et al. 2011). The study of any single parameter may not give adequate results and does not show a clear picture of alterations induced by pollutants. Thus, APTI as a complex index is especially useful to assess the tolerance level of any plant species based on their physiological and biochemical parameters (Das and Prasad 2010). It provides a reliable and reproducible method for screening sensitive and tolerant plant species under field conditions, where air is contaminated with various pollutants (Liu and Ding 2008).
The aim of our study was to investigate element concentration, dust content (PM10), and air pollution tolerance index (APTI) in various plant leaves along an urbanisation gradient in Vienna, Austria. Leaves of Fraxinus excelsior L. (common ash), Quercus robur L. (European oak), and Acer platanoides L. (Norway maple) were studied; these tree species are native and widely distributed in Europe (https://www.i-flora.com/en.html). Our hypotheses were as follows: (i) there are significant differences in pollutant levels among the different stages along the urbanisation gradient (urban, suburban, and rural areas). (ii) The highest metal and dust concentration is in the urban area, while the lowest is in the rural area; (iii) The tree species differ in sensitivity to air pollution, and this is reflected by their APTI values.
Material and methods
Study sites
Sampling locations were in and around the city of Vienna, Austria. Vienna is the major administrative and cultural center of Austria. The main pollution source is vehicular emissions, caused by multiple roads and highways with heavy traffic. We studied the effects of urbanisation on air pollution levels along an urbanisation gradient. Urban, suburban, and rural areas were studied (Fig. 1).
Sampling areas in Vienna, Austria (Bibi et al. 2023)
Sample collection
We collected leaves (N = 150) of F. excelsior, Q. robur and A. platanoides species along an urbanisation gradient during third week of August 2021. The trees were healthy, and neither pests nor symptoms of disease were found by visual examination. The assessed age of individuals was about 10–15 years. The sampling sites were located in an urban area (a busy park in the city center), a suburban area, and a forested rural area. We randomly chose 5 tree specimens at each sampling site, and we collected 30 leaves from each tree specimen. Samples were stored in paper bags and placed in the refrigerator at -21 °C.
Dust amount analysis
The surface area of sampled leaves was measured by scanning in black and white, and the scanned pictures were analysed using Image software (Simon et al. 2014, 2016, 2021). Afterwards, leaves were put into 500 ml plastic containers with 250 ml of deionised water and placed on an orbital shaker for 10 min. The suspension was filtered through a 150 µm sieve. Then, the procedure was repeated with 50 ml deionised water. This 300 ml suspension was filtered through filter paper (retention diameter of 5–8 µm, Munktell 392, Ahlstrom) using a vacuum filter machine (N811KN.18 Laboport). Thus, coarse dust (PM10) could be measured using the gravimetric method. Filter papers were weighed before and after filtration to determine the amount of dust collected on the paper. The amount of dust was expressed in mass per area (µg cm−2) (Simon et al. 2014, 2016, 2021).
Air pollution tolerance index (APTI)
APTI calculation is based on the tree leaves’ ascorbic acid content in mg g−1 (A), total chlorophyll content in mg g−1 (T), pH of leaf extract (P), and relative water content (R). Using these parameters, APTI was calculated by the equation below (Singh et al. 1991):
Ascorbic acid content was measured by titration; 2 g of leaf tissue was crushed and homogenised with 50 ml water in 3–4 parts. The collected extract filled up to 100 ml in volumetric flasks. Leaf pH was measured from this extract using a digital pH meter. After pH measurement, 20 ml portions of the sample were titrated in triplicate with 0.0025 ml of iodine solution and 1 ml of 0.5% starch solution, until the blue color remained for 20 s. Chlorophyll was extracted from 20 mg of fresh leaf using 5 ml of 96% ethanol. The absorbance of the extracts was measured using spectrophotometric analysis at wavelengths of 653, 666, and 750 nm. The total chlorophyll content (T) was calculated as follows:
where V is the volume (ml) of the leaf extract, m is the fresh weight (g) of the leaf sample, and E666 and E653 are the absorbances at 666 nm and 653 nm minus the absorbance at 750 nm, respectively. To determine the relative water content, the fresh weight of individual leaves (FW) was measured. Then, the leaves were weighed again after being immersed in water overnight to determine the turgid weight (TW). Finally, the leaves were dried in an oven at 70℃ to determine the dry weight (DW). The relative water content (R) was calculated as follows:
The sensitivity of tree species based on APTI values is categorized as follows: APTI > 24—tolerant; 20–24—moderately tolerant; 15–19—intermediate; and ≤ 14—sensitive species (Singh et al. 1991).
Elemental analysis in leaves
Leaf samples were dried at 60 °C for 24 h after the dust measurement. Then, the samples were homogenised and stored in plastic tubes until further treatment. For elemental analysis, 0.2 g of plant tissue was digested using 5 ml 65% (m/m) nitric acid and 1 ml 30% (m/m) hydrogen peroxide. Digested samples were diluted to 25 ml with deionised water. Inductively coupled plasma optical emission spectrometry (ICP-OES 5110 Agilent Technologies) was used to measure the elemental contents of leaf samples (Simon et al. 2011, 2014, 2016, 2021). We used a six-point calibration procedure with a multi-element calibration solution (Merck ICP multi-element standard solution IV) and measured the concentration of Al, Ba, Ca, Cr, Cu, Fe, K, Mg, Mn, Na, Ni, Pb, and Zn. Peach leaves (1547) CRM were used, and the recoveries were within 10% of the certified values for the elements (Simon et al. 2014, 2016, 2021).
Statistical analysis
The homogeneity of variances was tested with Levene’s test. Differences among species and areas were tested using analysis of variances (ANOVA) for each variable. Turkey’s test was used for pairwise comparison between the groups. Canonical discriminant analysis (CDA) was used to identify those variables that most efficiently discriminated the study area as a dependent variable. Dust content, total chlorophyll content, ascorbic acid content, leaf pH, and relative water content were used as independent variables. We repeated the CDA with the elemental concentration in leaf tissues as independent variables to separate the study area as a dependent variable. Pearson correlation was used to explore the correlation between studied parameters and air pollutants’ concentration. PM2.5, PM10, CO, and NO2 air pollutant data were collected from the European Environment Agency webpage (https://www.eea.europa.eu).
Results
Differences along an urbanisation gradient
Based on all APTI parameters and the elemental concentration of A. platanoides, significant differences were found among urban, suburban, and rural areas using canonical discriminant analysis (CDA) (Fig. 2). APTI values showed no significant differences among urban, suburban, and rural areas for A. platanoides, but the APTI values of the species suggest that it is sensitive to air pollution (Fig. 3). Significant differences were found among the areas in the amounts of PM10 (F = 4.786, P = 0.030) and the concentrations of Al (F = 31.950, P < 0.001), Cr (F = 4.757, P = 0.030), Fe (F = 4.181, P = 0.042), and Mn (F = 4.181, P = 0.042). The highest amount of PM10 and the highest concentration of Cr were found in the suburban area. The highest Al and Fe concentrations were found in the urban area. The highest Mn concentration was found in the rural area (Supplementary Material Table 1).
Using CDA based on all APTI parameters and elemental concentration of F. excelsior, we found significant differences among areas (Fig. 4). No significant difference was observed among areas based on the APTI value of F. excelsior; it is also a sensitive species (Fig. 5). We observed significant differences among the areas in the amounts of AAC (F = 25.043, P = 0.000), TChl (F = 4.286, P = 0.039), Ba (F = 7.629, P = 0.007), Cr (F = 4.922, P = 0.027), Cu (F = 2.817, P = 0.099), Fe (F = 3.240, P = 0.075), Sr (F = 3.662, P = 0.057), and Zn (F = 13.915, P = 0.001) in F. excelsior leaves. The highest AAC amount and Cu, Fe, and Zn concentrations were found in the urban area. The highest Ba and Cr concentrations were found in the suburban area. The highest TChl amount and Sr concentration was found in the rural area (Supplementary Material Table 2).
Based on all APTI parameters and the elemental concentration of Q. robur, significant differences were found among areas using CDA (Fig. 6). Q. robur is a sensitive species. Significant differences were not found among areas based on APTI (Fig. 7). We did, however, find significant differences among the areas in the amounts of TChl (F = 9.101, P = 0.004), Al (F = 4.542, P = 0.034), Cr (F = 12.462, P = 0.001), Fe (F = 23.159, P = 0.000), Mn (F = 5.353, P = 0.022), Ni (F = 20.651, P = 0.000), and Sr (F = 3.580, P = 0.060) in Q. robur leaves. The highest TChl amount and Al, Cr, and Fe concentrations were found in the urban area. The highest Sr concentration was found in the suburban area. The highest Mn and Ni concentrations were found in the rural area (Supplementary Material Table 3).
Differences among the species
We found significant differences among species based on all APTI parameters and elemental concentrations using CDA within all studied area (Figs. 8, 9, 10). We also found significant differences among the species in the urban area in the amounts of RWC (F = 7.799, P = 0.007), pH (F = 5.833, P = 0.017), ACC (F = 8.134, P = 0.006), TChl (F = 17.666, P < 0.001), APTI (F = 3.384, P = 0.068), Al (F = 30.118, P < 0.001), Cr (F = 6.222, P = 0.014), Cu (F = 6.182, P = 0.014), Fe (F = 18.207, P = 0.000), Mn (F = 8.006, P = 0.006), Ni (F = 19.289, P < 0.001), and Sr (F = 21.348, P < 0.001). The highest TChl and APTI amounts and Al, Fe, and Mn concentrations were found in Acer leaves from the urban area. The highest RWC amount and Cu, Ni, and Sr concentrations were found in the leaves of Fraxinus from the urban area. The highest pH and ACC amounts and Cr concentration were found in the Quercus leaves from the urban area (Supplementary Material Table 1– 3).
Significant differences were found among the species in the amounts of RWC (F = 15.883, P = 0.000), pH (F = 17.182, P < 0.001), ACC (F = 17.044, P < 0.001), TChl (F = 3.956, P = 0.048), APTI (F = 17.089, P < 0.001), PM10 (F = 4.856, P = 0.028), Al (F = 8.510, P = 0.005), B (F = 3.687, P = 0.056), Ba (F = 13.002, P = 0.001), Cr (F = 3.010, P = 0.087), Fe (F = 12.535, P = 0.001), Mn (F = 6.135, P = 0.015), Ni (F = 4.146, P = 0.048), Sr (F = 5.585, P = 0.019), and Zn (F = 15.377, P = 0.001) in the Kurpark suburban area. The highest RWC, pH, TChl, APTI, PM10 amounts and Al, B, Cr, Fe, and Mn concentrations were found in Acer leaves from the suburban area. The highest Ba, Ni, and Sr concentrations were found in Fraxinus leaves from the suburban area. Additionally, the highest pH and AAC amounts and the highest Zn concentration were found in Quercus leaves from the suburban area (Supplementary Material Table 1–3).
We found significant differences among the species in the rural area in the amounts of RWC (F = 13.417, P = 0.001), pH (F = 11.872, P = 0.001), ACC (F = 5.370, P = 0.022), APTI (F = 5.399, P = 0.012), B (F = 10.813, P = 0.002), Ba (F = 5.399, P = 0.024), Cr (F = 4.800, P = 0.029), Mn (F = 3.542, P = 0.062), Sr (F = 22.492, P < 0.001), and Zn (F = 11.527, P = 0.002). The highest pH and APTI values and B, Cr, Mn, and Zn concentrations were found in Acer leaves from the rural area. The highest RWC amount and Ba and Sr concentrations were found in Fraxinus leaves from the rural area. Additionally, the highest pH and AAC amounts were found in Quercus leaves from the rural area (Supplementary Material Table 1–3).
Correlation between studied parameters and air pollutants
For all studied species, we found a positive correlation between all studied parameters and annual NO2 and PM2.5 averages (Supplementary Table 4). For Q. robur, we also found a significant positive correlation between Cr concentration and annual NO2 and PM2.5 averages. For A. pseudoplatanus and F. excelsior, we found a positive correlation between Cu concentration and annual NO2 and PM2.5 averages (Supplementary Material Table 4).
Discussion
Our findings suggest that urban tree leaves can be used to effectively monitor urban air quality. Leaves trap dust and air pollutants due to specific features in the anatomy of their tissues, such as trichomes and stomata density. Tree species showed significant differences in relative moisture content, pH, ascorbic acid, and chlorophyll content. We found that all three species are sensitive to air pollution, based on APTI values. APTI values showed no significant differences among areas along the urbanisation gradient for A. platanoides, F. excelsior, and Q. robur. Based on the statistical evaluation, we found significant differences in relative moisture content, pH, ascorbic acid, and chlorophyll content among the different species. We also found a significant difference among areas in chlorophyll content and pH. In Q. robur, we observed a significantly lower relative moisture content, and in F. excelsior, we observed a significantly lower ascorbic acid content. The highest chlorophyll content was found in A. platanoides. There were significant differences in the Al, B, Ba, Cr, Fe, Mg, Mn, Na, Ni, P, S, and Zn concentrations in leaves among the species.
A. platanoides is the most prevalent species in cities throughout Europe, the USA, and Australia, and it is spreading rapidly throughout the world. A. platanoides trees were previously chosen primarily for their aesthetic qualities and urban adaptable characteristics, rather than for biomonitoring (McKinney 2006). Krzyżaniak et al (2015) reported that A. platanoides appears to be the best option for city parks, given its tolerance of various anthropogenic forces. Additionally, F. excelsior is an excellent choice for urban landscaping, due to its high endurance of adverse environmental conditions. Even in experimental plots with high and extremely high levels of pollution, this species maintains a reasonable level of biochemical markers (Petrova et al. 2022). Simon et al. (2014) demonstrated that Q. robur were useful biological indicators because of their large stomata size and high stomata density. Q. robur also showed a good correlation with scanning electron-microscopic findings. Arend et al. (2011) and Prusty et al. (2005) described the differences in deposited dust amounts based on different epidermal characteristics and leaf structures.
Alotaibi et al. (2020) reported significant reduction of leaf area in P. accerifolia in contaminated areas. The differences in the reduction in leaf areas between these species at different locations may be due to the leaves’ capacity to capture air pollutants (Haynes et al. 2019). Our results were similar to Simon et al. (2021); APTI values demonstrated moderate tolerance for A. saccharinum and intermediate tolerance for P. accerifolia. We observed significant differences among the species in the ascorbic acid content, the pH, and the total chlorophyll content of leaves. Furthermore, we found significant differences among the species in the Al, Ba, Ca, Fe, Mg, Ni, S, Sr, and Zn concentrations of leaves. Similar results were obtained by Rai et al. (2013), who reported that plants’ tolerance of air pollution may be site-specific, because Ficus bengalensis was tolerant in industrial sites and Mangifera indica was tolerant in non-industrial sites. Ogunkunle et al. (2015) suggested that the integration of plant tolerance and performance indices for the selection of tree species is useful to develop of a green belt using APTI values. According to Jyothi and Jaya (2010), sensitive tree species can serve as indicators for the reduction of air pollution, while tolerant tree species can act as sinks. Additionally, Gholami et al. (2016) discovered that plants with higher APTI values can be utilised to lower air pollution, while those with lower APTI values can be used to evaluate air pollution.
Conclusions
We assessed the usefulness of tree species in urban green area planning in relation to pollution tolerance and bioindication of air pollution using APTI, PM10, and heavy metal contents. We demonstrated that APTI is useful for selecting pollution-tolerant species, which can be used for urban green infrastructure planning. We found that, based on APTI values, A. platanoides, F. excelsior, and Q. robur were sensitive indicator species of air pollution. Tree species showed significant differences in their leaves’ relative moisture content, pH, ascorbic acid, and chlorophyll content. We found significant differences among the studied areas along the urbanisation gradient in chlorophyll content and pH. Among the species, we observed significant differences in the Al, B, Ba, Cr, Fe, Mg, Na, Ni, P, S, and Zn concentrations of leaves. We demonstrated that tree leaves are reliable bioindicators of urban air pollution. Our findings showed the effect of urbanisation on these bioindicator species and their parameters, as higher PM10, Al, Ba, Cu, Fe, Sr, and Zn concentrations found in the urban area than in the rural area.
Data Availability
Data available on author’s request.
References
Alotaibi MD, Alharbi BH, Al-Shamsi MA, Alshahrani TS, Al-Namazi AA, Alharbi SF, Alotaibi FS, Qian Y (2020) Assessing the response of five tree species to air pollution in Riyadh City, Saudi Arabia, for potential green belt application. Environ Sci Pollut Res 27:29156–29170. https://doi.org/10.1007/s11356-020-09226-w
Arend KK, Beletsky D, De Pinto JV, Ludsin SA, Roberts JJ, Rucinski DK, Scavia D, Schwab DJ, Höök TO (2011) Seasonal and interannual effects of hypoxia on fish habitat quality in central Lake Erie. Freshw Biol 56:366–383. https://doi.org/10.1111/j.1365-2427.2010.02504.x
Bibi D, Tőzsér D, Sipos B, Tóthmérész B, Simon E (2023) Heavy metal pollution of soil in Vienna, Austria. Water Air Soil Pollut 234:232. https://doi.org/10.1007/s11270-023-06244-5
Çelik A, Kartal AA, Akdoğan A, Kaska Y (2005) Determining the heavy metal pollution in Denizli (Turkey) by using Robinio pseudo-acacia L. Environ Int 31:105–112. https://doi.org/10.1016/j.envint.2004.07.004
Das S, Prasad P (2010) Seasonal variation in air pollution tolerance indices and selection of plant species for industrial areas of Rourkela. Indian J Environ Prot 30:978–988
Gholami A, Mojiri A, Amini H (2016) Investigation of the Air Pollution Tolerance Index (APTI) using some plant species in Ahvaz region. J Anim Plant Sci 26:475–480
Haynes A, Popek R, Boles M, Paton-Walsh C, Robinson SA (2019) Roadside moss turfs in South East Australia capture more particulate matter along an urban gradient than a common native tree species. Atmosphere 10:224. https://doi.org/10.3390/atmos10040224
Hueglin C, Gehrig R, Baltensperger U, Gysel M, Monn C, Vonmont H (2005) Chemical characterisation of PM2.5, PM10 and coarse particles at urban, near-city and rural sites in Switzerland. Atmos Environ 39:637–651. https://doi.org/10.1016/j.atmosenv.2004.10.027
Jyothi SJ, Jaya DS (2010) Evaluation of air pollution tolerance index of selected plant species along roadsides in Thiruvananthapuram, Kerala. J Environ Biol 31:379–386
Kardel F, Wuyts K, Babanezhad M, Vitharana UWA, Wuytack T, Potters G, Samson R (2010) Assessing urban habitat quality based on specific leaf area and stomatal characteristics of Plantago lanceolata L. Environ Pollut 158:788–794. https://doi.org/10.1016/j.envpol.2009.10.006
Krzyżaniak M, Świerk D, Walerzak M, Urbański P (2015) The impact of urban conditions on different tree species in public green areas in the city of Poznan. Folia Hortic 27:89–97. https://doi.org/10.1515/fhort-2015-0018
Kuddus M, Kumari R, Ramteke PW (2011) Studies on air pollution tolerance of selected plants in Allahabad city, India. J Environ Res Manage 2:042–046
Larkin A, van Donkelaar A, Geddes JA, Martin RV, Hystad P (2016) Relationships between changes in urban characteristics and air quality in East Asia from 2000 to 2010. Environ Sci Technol 50:9142–9149. https://doi.org/10.1021/acs.est.6b02549
Liu YJ, Ding HUI (2008) Variation in air pollution tolerance index of plants near a steel factory: Implication for landscape-plant species selection for industrial areas. WSEAS Trans Environ Dev 4:24–32
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Conserv 127:247–260. https://doi.org/10.1016/j.biocon.2005.09.005
Molnár VÉ, Tőzsér D, Szabó S, Tóthmérész B, Simon E (2020) Use of leaves as bioindicator to assess air pollution based on composite proxy measure (APTI), dust amount and elemental concentration of metals. Plants 9:1743. https://doi.org/10.3390/plants912174
Ogunkunle CO, Suleiman LB, Oyedeji S, Awotoye OO, Fatoba PO (2015) Assessing the air pollution tolerance index and anticipated performance index of some tree species for biomonitoring environmental health. Agrofor Syst 89:447–454. https://doi.org/10.1007/s10457-014-9781-7
Petrova S, Velcheva I, Nikolov B, Vasileva T, Bivolarski V (2022) Antioxidant responses and adaptation mechanisms of tilia tomentosa moench, Fraxinus excelsior L. and Pinus nigra J.F. Arnold towards urban air pollution. Forests 13:1689. https://doi.org/10.3390/f13101689
Prusty BAK, Mishra PC, Azeez PA (2005) Dust accumulation and leaf pigment content in vegetation near the national highway at Sambalpur, Orissa, India. Ecotoxicol Environ Saf 60:228–235. https://doi.org/10.1016/j.ecoenv.2003.12.013
Rai PK, Panda LL, Chutia BM, Singh MM (2013) Comparative assessment of air pollution tolerance index (APTI) in the industrial (Rourkela) and non industrial area (Aizawl) of India: An ecomanagement approach. Afr J Environ Sci Technol 7:944–948
Simon E, Braun M, Vidic A, Bogyó D, Fábián I, Tóthmérész B (2011) Air pollution assessment based on elemental concentration of leaves tissue and foliage dust along an urbanization gradient in Vienna. Environ Poll 159:1229–1233. https://doi.org/10.1016/j.envpol.2011.01.034
Simon E, Baranyai E, Braun M, Cserháti C, Fábián I, Tóthmérész B (2014) Elemental concentrations in deposited dust on leaves along an urbanization gradient. Sci Total Environ 490:514–520. https://doi.org/10.1016/j.scitotenv.2014.05.028
Simon E, Harangi S, Baranyai E, Fábián I, Tóthmérész B (2016) Influence of past industry and urbanization on elemental concentrations in deposited dust and tree leaf tissue. Urban For Urban Green 20:12–19. https://doi.org/10.1016/j.ufug.2016.07.017
Simon E, Molnár VÉ, Domonkos L, Bibi D, Tóthmérész B, Sz Szabó (2021) Usefulness of tree species as urban health indicators. Plants 10:2797. https://doi.org/10.3390/plants10122797
Singh SK, Rao DN, Agrawal M, Pandey J, Naryan D (1991) Air pollution tolerance index of plants. J Environ Manage 32:45–55. https://doi.org/10.1016/S0301-4797(05)80080-5
Wang S, Zhou C, Wang Z, Feng K, Hubacek K (2017) The characteristics and drivers of fine particulate matter (PM2.5) distribution in China. J Clean Prod 142:1800–1809. https://doi.org/10.1016/j.jclepro.2016.11.104
Funding
Open access funding provided by University of Debrecen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This research doesn’t involve human and animal subjects.
Consent to participate
This research doesn’t involve human subjects to participate in the study.
Consent to publish
This research doesn’t involve human research participants.
Competing interests
The authors declare that they have no know competing interests or personal relationships that could have appeared to influence the work reported in this paper. This project has received funding from the HUN-REN Hungarian Research Network.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bibi, D., Tőzsér, D., Sipos, B. et al. Complex study of air pollution based on tree species in Vienna. Air Qual Atmos Health 17, 417–424 (2024). https://doi.org/10.1007/s11869-023-01452-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-023-01452-8