Abstract
The oxidative potential (OP) is defined as the ability of inhaled PM components to catalytically/non-catalytically generate reactive oxygen species (ROS) and deplete lung antioxidants. Although several studies have measured the OP of particulate matter (PM OP) soluble components using different antioxidants under neutral pH conditions, few studies have measured PM OP with acidic lung fluids. This study provides new insights into the use of acidic rather than neutral fluids in OP assays. Thus, the first aim of this study was to clarify the effect of using an acidic lung fluid on ascorbic acid (AA) depletion. This was achieved by measuring the oxidative potential (OP-AA) of individual compounds known to catalyze the AA oxidation (CuSO4, CuCl2, and 1,4-NQ) in artificial lysosomal fluid (ALF, pH 4.5), a commonly used acidic simulated lung fluid, and in a neutral fluid (phosphate-buffered saline (PBS1x), pH 7.4). Our results from these individual compounds showed a significant decrease of OP-AA in the acidic fluid (ALF) with respect to the neutral fluid (PBS). Then, the second aim of this work was to investigate whether the OP-AA assay could be applied to PM2.5 samples extracted in acidic conditions. For this purpose, OP-AA and bioaccessible concentrations of metal(loid)s (V, Mn, Fe, Ni, Cu, Zn, As, Mo, Cd, Sb, and Pb) of PM2.5 samples collected in an urban-industrial area that were extracted in ALF were analyzed. The mean volume-normalized OP (OP-AAv) value was 0.10 ± 0.07 nmol min−1 m−3, clearly lower than the values found in the literature at neutral pH. OP-AAv values were highly correlated with the ALF-bioaccessible concentration of most of the studied metal(loid)s, mainly with Cu and Fe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exposure to particulate matter (PM) has been significantly linked with cardio-respiratory diseases in numerous epidemiological studies (Kim et al. 2015; Shiraiwa et al. 2017; Pope et al. 2020). PM toxicity has been strongly related to its chemical composition, to its size (Kelly and Fussell 2012), and in particular to its ability to cause oxidative stress in the exposed population (Weichenthal et al. 2013; Ristovski et al. 2012; Bates et al. 2019). Regarding their size, particles smaller than 2.5 μm (PM2.5) pose the greatest risk to health because they are mostly transported to the alveolar region (Mukhtar and Limbeck 2013). Furthermore, the most toxic components of PM are transition metals and certain organic compounds, such as polycyclic aromatic hydrocarbons (PAHs), quinones, and phthalates (Seinfeld and Pandis 2016).
Although the mechanisms of action for PM and its compounds on health are still not completely understood, extensive literature has pointed out that oxidative stress is likely one of the most important mechanisms of PM inhalation toxicity (Ghio et al. 2012; Weichenthal et al. 2013; Møller et al. 2014). Oxidative stress corresponds to an imbalance between the generation of reactive oxygen species (ROS) and the antioxidant defenses in cells and tissues (Sauvain et al. 2014; Tian et al. 2017). The ROS can be exogenously introduced to the body via particle inhalation and/or generated in vivo inside the cell via cellular redox reactions stimulated by target inhaled PM compounds (Bates et al. 2019; Park et al. 2018). An excess of ROS also increases the inflammatory response, which can cause various diseases, including respiratory and cardiovascular conditions (He et al. 2018; Van Den Heuvel et al. 2016).
In recent years, the oxidative potential (OP)—defined as the ability of inhaled PM components to catalytically/non-catalytically generate ROS and deplete lung antioxidants (Bates et al. 2019; Øvrevik 2019)—has been suggested as a relevant exposure metric to predict PM toxicity. Extensive research has measured PM OP using multiple acellular assays, among which the most widely used are the dithiothreitol (OP-DTT) and the ascorbic acid (OP-AA) tests (Øvrevik 2019; Gao et al. 2020a; Clemente et al. 2023). Nonetheless, there are many protocols for measuring OP within each assay and considerable doubts regarding which assay parameters should be adopted (Hedayat et al. 2015; Bates et al. 2019; Shen et al. 2021; Song et al. 2021).
Recent toxicological research has broadly assumed that the solubility of PM-bound compounds in pulmonary fluids (bioaccessibility) is one of the key factors for toxicological assessments from PM (Wallenborn et al. 2007; Charrier and Anastasio 2012; Mukhtar and Limbeck 2013; Guo et al. 2019). Several studies have measured PM OP when particles are extracted with ultrapure water followed by filtration to remove the insoluble fraction (Fang et al. 2016; Pietrogrande et al. 2018; Khoshnamvand et al. 2023). However, the use of water as a leaching agent is unrepresentative of physiological conditions in the lungs. For this reason, there is a growing number of investigations in which PM is extracted with simulated lung fluids (SLFs) frequently applied in bioaccessibility tests (Calas et al. 2019; Moufarrej et al. 2020; Pietrogrande et al. 2021; Song et al. 2021).
To assess metal(loid) bioaccessibility in the fine fraction of PM, two SLFs are commonly used in the literature: Gamble’s solution (pH 7.4) and artificial lysosomal fluid (ALF) (pH 4.5). Gamble’s solution is representative of the neutral interstitial fluid of the deep lung, whereas ALF represents the more acidic intracellular conditions found in the lysosomes of alveolar macrophages (Colombo et al. 2008; Mukhtar and Limbeck 2013; Innes et al. 2021).
Several operating factors, such as extraction solvents, extraction techniques, and filter types, have been investigated in OP evaluation (Calas et al. 2017; Frezzini et al. 2022; Khoshkam et al. 2022) and bioaccessibility tests as well (Colombo et al. 2008; Pelfrêne et al. 2017; Expósito et al. 2021a). Nevertheless, OP assay conditions have not been fully studied (Li et al. 2009; Shen et al. 2021); in particular, the composition of the leaching agent and the influence of the pH on the reactions involved in OP measurement still need to be thoroughly investigated (Øvrevik 2019; Shahpoury et al. 2019; Wei et al. 2021).
Some studies have compared PM OP at neutral pH when water, ALF, and Gamble’s solution are used in the extraction (Calas et al. 2017; Cigánková et al. 2021; Pietrogrande et al. 2021). However, to our knowledge, only two recent studies have measured OP-AA under acidic conditions after extracting PM with an acidic simulated lung fluid (ALF) (Schiavo et al. 2022; Wu et al. 2022). Only Wu et al. (2022) measured the OP-AA of PM2.5 samples; however, this study did not focus on discussing the lower reactivity observed in ALF compared to neutral fluids, nor on evaluating the sensitivity of OP-AA test in ALF to the concentrations of PM compounds and the emission sources.
The OP values were found to depend on the acidity and the composition of the extraction solution and on the soluble metal concentration. On one hand, previous investigations on metal(loid) bioaccessibility have shown that an increase in the solubility of toxic pollutants from PM occurs in acidic media, e.g., bioaccessibility in ALF is higher than in Gamble’s solution (Colombo et al. 2008; Hernández-Pellón et al. 2018; Expósito et al. 2021a; Innes et al. 2021). A higher metal(loid) concentration in the OP assay would lead to a higher OP value. However, on the other hand, former research has highlighted that the ascorbate test for catalytic metals is more effective at neutral pH than at acidic pH because the oxidation of ascorbic acid catalyzed by metals is too slow at low pH. As a result, some investigations such as Gosselin and Zagury (2020) decided only to use Gamble’s solution in the OP-AA assay because of the higher sensitivity of this assay near neutral pH, although both Gamble’s solution and ALF were used to extract PM components in bioaccessibility tests. In fact, ascorbic acid species depend on pH (pka1 4.12, pka2 11.5) (Du et al. 2012), and the OP-AA assay follows the depletion of the ascorbate anion, so its concentration at acidic pH is lower (Weissberger et al. 1943; Khan and Martell 1967; Buettner 1988; Shen et al. 2021). Besides, the composition of SLFs may affect the OP, because species such as chloride, citrate, and glycine, which are found in some SLFs, could also reduce or inhibit the loss of AA (Mapson 1945; Ayres et al. 2008; Pietrogrande et al. 2019; Shahpoury et al. 2019). Therefore, OP assays should be further investigated in acidic SLFs, since only a few studies have recently reported OP-AA values in these conditions, and poor or null discussion on why increased acidity modifies the PM OP can be found in such references.
It is important to highlight that under a macrophage attack scenario, the leaching of PM-bound metal(loid)s would occur in acidic conditions (Roth 2006; Mukhtar and Limbeck 2013); once solubilized, these metal(loid)s would contribute to the antioxidant depletion.
The purpose of this work was to assess the ascorbic acid PM OP in acidic simulated lung fluid. First, individual compounds known to catalyze the AA oxidation (CuSO4, CuCl2, and 1,4-NQ) in an acidic fluid (ALF, pH 4.5) were considered, and their OP was measured and compared with that obtained with a neutral fluid (phosphate-buffered saline (PBS1x), pH 7.4). Then, the OP-AA assay in ALF media was applied to PM2.5 sampled in an urban-industrial area (Maliaño, Northern Spain). ALF-bioaccessible concentrations of metal(loid)s (V, Mn, Fe, Ni, Cu, Zn, As, Mo, Cd, Sb, and Pb) in PM2.5 samples were also measured in order to associate the OP-AA values in acidic conditions with the metal(loid) bioaccessible contents.
Methodology
PM2.5 sampling campaign
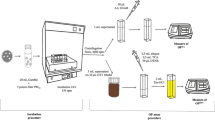
A PM2.5 sampling campaign was conducted in the north of Spain, in the region of Cantabria located alongside the southern part of the Santander Bay, specifically in Maliaño. This area is mainly characterized by high air Mn concentrations, identifying the presence of a ferromanganese alloy industry as the main source of Mn (Arruti et al. 2011; Ruiz et al. 2014). The sampling site was located on the rooftop of the “Cultural Center of La Vidriera” (latitude: 43° 24′ 39.6″ N; longitude: 3° 50′ 13.2″ E) some 350m north of a Mn alloy production plant (Fig. 1). The sampling site is located downwind of the Mn alloy plant based on our earlier studies (Otero-Pregigueiro and Fernández-Olmo 2018; Hernández-Pellón and Fernández-Olmo 2019). A more detailed description of the sampling site can be found in Hernández-Pellón and Fernández-Olmo (2019).
PM2.5 samples were collected using an impactor (Dekati® PM10 Impactor, PM2530) with a flow rate of 30 L/min for 24 h on a 47 mm Teflon filter. Overall, 60 PM2.5 daily samples were collected at the Vidriera site, 34 from November 2019 to March 2020, and 26 from September to November 2020 (the sampling campaign was interrupted due to the COVID-19 lockdown).
Once the gravimetric determination was performed, the filters were cut into two pieces using ceramic scissors. Half of the filter was used for the OP-AA analysis, while the other half was used for the determination of the metal(loid) bioaccessible concentrations.
Standard solutions and reagents
All chemicals were of analytical grade from AppliChem GmbH and Sigma-Aldrich and all solutions were prepared in purified water from a Milli-Q system (Millipore, ≥18.2 MΩ cm). ALF (pH 4.5±0.1) was daily freshly prepared using ultrapure water and mixing the stock solutions following the order given in Table S1. The pH of the ALF was adjusted to 4.5 by adding dropwise 1 M of nitric acid (HNO3) or sodium hydroxide (NaOH). Besides, phosphate-buffered saline x1 (PBS1x, 0.01M phosphate buffer, 0.154M sodium chloride) at pH 7.4±0.1 was prepared by diluting phosphate-buffered saline x10 (PBS10x, 0.1M phosphate buffer, 1.5M sodium chloride).
Standard solution of ascorbic acid was prepared at a concentration of 10 mM in ultrapure water and stored at −20°C in an amber glass vial due to its instability at room temperature until the measurement of PM OP values.
Three compounds known to catalyze the AA oxidation were considered: 1,4-naphthoquinone (1,4-NQ), copper (II) sulfate (CuSO4), and copper (II) chloride (CuCl2). Individual standard stock solutions were prepared at a concentration of 0.01 M using ultrapure water for metal salts and methanol for 1,4-NQ as solvents. Then, aliquots of Cu stock solutions were diluted in PBS1x (pH 7.4) and ALF (pH 4.5) at environmentally relevant concentrations (Charrier and Anastasio 2012; Visentin et al. 2016; Pietrogrande et al. 2022a), leading to final Cu concentrations in the microplate wells in the range of 0.34 to 1 μM, while the final concentration of 1,4-NQ was in the range reported by Calas et al. (2018) and Moufarrej et al. (2020), when it was used as a positive control (8 μM).
Ascorbic acid oxidative potential assay
The OP-AA assay was performed following the procedure of Moufarrej et al. (2020), but using ALF as a leaching agent.
PM2.5 sample extraction in ALF
Briefly, for each PM2.5 sample, half of the filter was incubated with 4.5 mL of ALF in a heated orbital shaker for 24 h at 37°C, at a speed of 250 oscillations/min. Solutions were then filtered through 0.45-μm nylon filters, and the filtrates were placed in flasks and stored at 4°C until OP analysis. The maximum storage time was 24h.
Determination of ascorbic acid oxidative potential
The OP-AA assay was performed in 96 well plates with UV-transparent flat bottom. 160 μL of PM leachate (or blank or pure compound solution) was placed in each well of the plate, and the latter was incubated at 37°C for 5 min. Then, 40 μL of 1mM AA solution was added into the wells (so that the final AA concentration was 200 μM) and was shaken for 1 min. Finally, the microplate was placed in the spectrophotometer (Multiskan Go, Thermo Fisher Scientific, Finland) at 37°C, and the absorbance was measured at 265 nm every 2 min for 2h with a 30 s shaking step before each measurement. The OP-AA assay only accounts for the oxidation of ascorbate anion (AH−), since this species shows an absorbance peak at this wavelength. Note that the oxidation rate of the acid form (AH2) is much slower than that of AH− (Macartney and Sutin 1983). Prior to the absorbance measurements of chemical species and PM2.5 samples, calibration curves were made plotting the absorbance/concentration data of the calibration standards (200, 150, 100, 50, and 0 μM) in ALF or PBS1x.
The AA depletion rates (OP-AA value) of chemical species and PM2.5 samples were determined by considering the slope of the linear regression of the remaining AA versus time. In general, a good linearity was found for all samples with a correlation coefficient (R2) higher than 0.98 between 5 and 60 min. Samples were analyzed in triplicate (RSD<15%). The OP-AA values were expressed per unit of mass of PM (OP-AAm in pmol min−1 μg−1) and per unit of air volume (OP-AAv in nmol min−1 m−3).
For the detection limit (D.L.) calculation, OP-AA values of 18 half-filters were determined by measuring the depletion rates of AA after extracting laboratory blank filters in ALF. The D.L. was calculated by multiplying the standard deviation of the OP-AA values for the 18 laboratory blank filters with the n-1 sample two-tailed Student’s t-test at 95% confidence level (2.11). Furthermore, the OP-AA arithmetic mean of laboratory blank filters was subtracted from the depletion rate of each PM2.5 sample. In particular, the mean value to be subtracted was 0.034 μM/min and the D.L. was 0.091 μM/min. The slope of AA depletion curves for most of PM2.5 filters could be distinguished from that of blank filters, as shown in Figure S1. For statistical calculations, values below the D.L. were assigned D.L./2.
Bioaccessible metal(loid) concentration analysis in ALF
The metal(loid) bioaccessibility test was performed using ALF as leaching agent. Half of each PM2.5 filter was extracted with 10 mL ALF in an end-over-end rotation incubator system for 24h at 30 rpm and at 37°C using polypropylene (PP) tubes. The L/S ratios varied between 11,765 and 200,000. After the extraction, the samples were centrifuged at 4200 rpm for 10 minutes, and the supernatants were filtered through a 0.45-μm polypropylene syringe filter. The samples were stored until analysis at 4°C, and the maximum storage time was 48h.
Concentrations of 11 metal(loid)s (V, Mn, Fe, Ni, Cu, Zn, As, Mo, Cd, Sb, and Pb) in ALF were measured by inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7500 CE). Blanks were considered by treating 10 laboratory blank filters with the same protocol applied to PM2.5 filters. The arithmetic mean of blanks for each metal was subtracted from each measurement. D.L.s of the analyzed metal(loid)s were calculated based on the variability of the results of the 10 filter blanks (two-tailed Student’s t-test with 95% confidence for n-1 samples (2.26) times the standard deviation of the blanks). Values below the D.L. were replaced with D.L./2 for the statistical analysis. The D.L. values of metal(loid)s are shown in Table S2. Further details of ICP-MS analysis of bioaccessible metal concentrations are described elsewhere (Expósito et al. 2021a).
Statistical analysis
Statistical analysis of the data was performed using IBM SPSS Statistics software (version 22). Relationships between OP-AAv values and bioaccessible concentrations of metal(loid)s were investigated by calculating correlation coefficients and principal component analysis (PCA).
All data distributions were checked for normality using the Kolmogorov-Smirnov test. Since most distributions deviated from the normality, the association between bioaccessible concentrations of metal(loid)s and OP-AA values was evaluated by determining Spearman’s correlation coefficients (rho). A probability level of p < 0.05 was chosen to establish statistical significance.
Before applying the PCA, the data was first transformed into a dimensionless standardized form to consider the variability of the data set using the following equation:
where x is the daily bioaccessible concentration of the metal(loid) or OP-AAv, X is the arithmetic mean, and α is the standard deviation. Varimax orthogonal rotation was performed on the resolved factors in order to facilitate the interpretation. Resolved factors with high eigenvalues compared to the unity were considered to have a significant contribution. Additionally, the Kaiser-Meyer-Olkin (KMO) value was above 0.6 and the Bartlett value was found to be significant (p < 0.01).
Results and discussion
Behavior of pure compounds in neutral and acidic fluids
Figure 2 compares the AA depletion curves of 1,4-NQ, CuCl2, and CuSO4 over 60 minutes in ALF and PBS1x. The OP-AA values calculated from the depletion curves are shown in Table 1.
Our results show a strong decrease in the AA consumption in ALF with respect to PBS1x. Thus, high OP-AA values were obtained in PBS1x for 1,4-NQ (3.34 μM/min), similar to the value obtained by Moufarrej et al. (2020) (2.74 μM/min), but higher than that obtained by Visentin et al. (2016) at lower concentration of 1,4-NQ (0.64 μM/min at 1 μM). Our OP-AA values with Cu salts in PBS1X (1.29–1.33 μM/min at 1 μM) were lower than those obtained by Visentin et al. (2016) in PB solution (without chloride), 3.52 μM/min, at 0.17 μM Cu concentration. The role of chloride in the reduction or inhibition of copper activity has been studied in the literature. For instance, Pietrogrande et al. (2019) obtained higher OP-AA values when PB was used instead of PBS (2.33 vs 0.57 nmol/min). Although the mechanism of AA oxidation in the presence of chloride is still not well understood, Murekhina et al. (2022) suggested that the deceleration of ascorbic acid oxidation due to a higher concentration of chloride salts could possibly be explained by the formation of intermediate chloride complexes of Cu (I), whose oxidation rate differs from that of the free cation.
However, the AA consumption rate of 1,4-NQ in the acidic solution is so small that the slope cannot be distinguished from that of AA blank (0.08 μM min−1). This compound is usually used as a positive control in neutral media due to its high catalytic capacity in AA oxidation, but not in acidic solutions. Only the OP-AA of CuCl2 and CuSO4 could be calculated in ALF media. Similar values were obtained for the two Cu (II) salts. However, a sharp decrease in the OP-AA value in acidic pH with respect to neutral pH was observed for these salts, approximately a 60 % reduction both at 0.34 and 1 μM. The Cu concentration plays also an important role in the AA depletion rate, resulting in OP-AA values between 2.4 and 2.7 times higher when the Cu concentration increases from 0.34 to 1 μM, both in ALF and PBS1x.
These results agree well with the ascorbate depletion rate equation shown in the literature (Buettner 1988; Murekhina et al. 2022):
Thus, a higher concentration of the catalyst (Cu) leads to a higher depletion rate (OP-AA value) (Cervellati et al. 2020). In addition, the lower consumption rates of ascorbate in ALF compared to PBS1x could be partly explained by the variation of protonated AA species with pH. The AA assay measures the ascorbate species, which is dominant in neutral conditions and its amount decreases at lower pH while the amount of ascorbic acid species (AH2) increases (Khan and Martell 1967; Hayakawa et al. 1973; Buettner 1988). Specifically, the percentage of AH− is 71% and close to 100% at pH 4.5 and 7.4, respectively. This leads to a decrease in the initial AH− concentration at an acidic pH (see Fig. S2) leading to a lower reaction rate. However, the reduction in AA consumption in acidic conditions cannot be fully explained by the lower concentration of ascorbate species; the oxidation rate of ascorbate also decreases at acidic pH (Khan and Martell 1967), but this decrease depends on the catalyst (a much higher decrease with 1,4-NQ compared to Cu (II) as shown in Table 1). The lower depletion rate in ALF can also be explained by the effect of the fluid composition on the kinetic constant (kcat) of each catalyst. In particular, since ALF contains citrate, this may form complexes that affect the activity of the catalyst used. For example, Şahbaz and Somer (1993) reported that the rate of ascorbic acid oxidation depended on the catalytic activity of Cu(II)-citrate formed in the pH range of 2.5–6, following the order Cu(II)>H3CiCu+>HCiCu−>CiCu2−. However, 1,4-NQ was not expected to form strong complexes with the ALF components (Calas et al. 2017).
The results clearly show that the AA assay in PBS1x provides higher sensitive measurements of OP-AA than in ALF media. However, PBS1x is not a realistic representation of the conditions in the lungs and may overestimate the OP. ALF might better simulate the antioxidant depletion driven by PM compounds in biological conditions. In spite of the low sensitivity of OP assay in ALF media, the OP-AA of Cu (II), the most reactive ion reported in the bibliography (Khan and Martell 1967; Visentin et al. 2016), was accurately measured in ALF at a concentration range commonly found in ambient PM (Charrier and Anastasio 2012).
OP-AA of PM2.5 filters in an urban-industrial mixed site
The AA assay in ALF was applied to 60 PM2.5 filters collected at Vidriera site, obtaining 56 samples above the detection limit. This shows that the OP-AA assay was sensitive to quantify the OP levels even though the PM extraction and OP analysis were done in ALF. The OP-AA values ranged from a minimum of 0.09 μM min−1 to a maximum of 1.14 μM min−1. The mean air volume- and PM mass-normalized OP-AA values in PM2.5 were 0.10 ± 0.07 nmol min−1 m−3 and 13.6 ± 11.94 pmol min−1 μg−1, respectively (see Table 2). The PM2.5 OP-AA responses, both volume- and mass-normalized values, were far below than those observed in other urban and industrial areas (Fang et al. 2016; Moufarrej et al. 2020; Borlaza et al. 2021; Li et al. 2021; Pietrogrande et al. 2022b). For example, Moufarrej et al. (2020) reported an average AA depletion rate in PM2.5 samples solubilized in Gamble’s solution of 1.12 ± 1.50 nmol min−1 m−3 (OP-AAv) and 55 ± 66 pmol min−1 μg−1 (OP-AAm) in Dunkerque (France) and Pietrogrande et al. (2022b) reported a mean OP of PM2.5 samples solubilized in phosphate buffer of 1.08± 0.62 nmol min−1 m−3 air and 29± 100 pmol min−1 μg−1 in Milano Pascal (Italy). Similar values were also reported by Perrone et al. (2019) in Salento’s peninsula (Italy). However, all of these studies measured the OP-AA in neutral fluids. Only, Wu et al. (2022) obtained a similar OP mean value for PM2.5 samples collected in Shanghai (China) when ALF was used in the AA assay (0.33 ± 0.12 nmol min−1 m−3); this value was clearly lower than that obtained in PBS in the same study (1.6 ± 0.27 nmol min−1 m−3). It is important to highlight that the concentrations of metal(loid)s in ALF are higher than in neutral pH. However, the OP-AA values are lower proving a lower catalytic activity of PM2.5 components due to the pH and the composition of ALF.
Overall, the comparison of our results with the other studies supported the idea that lower value measurements of OP-AA are obtained when an acidic simulated lung fluid (ALF) is used in the extraction. In addition, the differences in the OP-AA values compared with other studies could also be caused by different ambient concentrations of PM2.5 components and the synergistic and antagonistic interactions among these components (Guo et al. 2020).
Bioaccessibility of PM2.5-bound metal(loid)s in ALF
The bioaccessible concentrations of PM2.5-bound metal(loid)s in ALF are shown in Table 2. The highest concentrations were found for Mn, Fe, Zn, and Pb. These concentration levels could be explained by the influence of the Mn alloy industry located in the sampling area (Hernández-Pellón et al. 2018; Hernández-Pellón and Fernández-Olmo 2019).
Mn is a metal of concern in the studied area. Although this metal is not regulated by EU air quality Directives, the World Health Organization (WHO) recommends an annual mean value of 150 ng m−3 as a guideline (WHO 2000). The mean bioaccessible concentration of Mn in PM2.5 at Vidriera site was 358 ng m−3 reaching daily values up to 1432.9 ng m−3, which exceeds by far the WHO guideline at this site. The mean Mn value is approximately three times higher than that obtained in a parallel sampling campaign carried out by personal samplers (mean: 109 ng m−3 for participants living <1.5 km from the same Mn alloy industry) (Expósito et al. 2021b). This difference can be explained by the different distances between the place of residence of the participants and the Mn alloy industry and the time spent indoors (Otero-Pregigueiro and Fernández-Olmo 2018; Markiv et al. 2022).
Spearman’s correlation coefficients between the analyzed metal(loid)s were calculated and are shown in Table 3. In addition, the PCA results are depicted in Table 4. The Varimax rotated solution provided a three-factor model with eigenvalues higher than 1, explaining nearly 65 % of the variance. The time profile of the metal(loid) levels is shown in Fig. 3 grouping them into the three factors obtained from PCA. The first factor explains 27% of the total variance and was assigned to the emissions from the Mn alloy plant located near the sampling site (Mn shows the highest factor loading followed by Zn, Cd, Pb, and Fe). In accordance with these results, strong Spearman’s correlation coefficients were found between these metals, Mn having correlation values of 0.86 with Pb and Cd, 0.74 with Fe, and 0.71 with Zn. These pollutants were also previously associated with this Mn alloy plant (Hernández-Pellón and Fernández-Olmo 2019). The second factor was attributed to non-exhaust road traffic emissions since Cu showed the highest factor loading, followed by Fe and Sb. These three metals are usually considered tracers of non-exhaust road traffic (Pant and Harrison 2013). Moderate correlations were also obtained for these elements (Table 3); the correlation coefficients of Fe-Cu, Fe-Sb, and Sb-Cu were 0.64, 0.55, and 0.65, respectively. The third factor is characterized by a high factor loading of Ni followed by V, Mo, and As. Previous studies in the same area linked Ni and V to ship exhaust emissions from the Santander Bay (Hernández-Pellón and Fernández-Olmo 2019), while the origin of As was not clearly identified (Hernández-Pellón and Fernández-Olmo 2019; Markiv et al. 2022). In addition, Ni showed the lowest correlations with the other elements. The interdependence of these groups of metal(loid)s (those related to the Mn alloy plant, non-exhaust road traffic emissions, and ship emissions) was also observed in Fig. 3, where similar time profiles in each group are shown, confirming the common origin of metal(loid)s in each group.
Association between OP-AA and metal(loid) bioaccessible concentrations in ALF
Spearman’s correlation coefficients between OP-AAv and ALF-bioaccessible concentrations of studied metal(loid)s in PM2.5 samples are shown in Table 3. Despite the low OP-AAv values because of the acidic conditions of the test, significant positive correlations were obtained between OP-AAv values and most of the studied metal(loid)s. The strongest correlations (rho>0.7) were obtained for Fe, Cu, As, and Sb, followed by Mn (rho=0.64). OP-AAv values showed moderate correlations with Zn, Mo, V, Cd, and Pb (0.5<rho<0.6) and a weak association with Ni. PCA results indicated that OP-AAv is mainly associated with the second factor (non-exhaust road traffic emissions) with a factor loading of 0.70. The first factor (ferroalloy plant emissions) also had a significant but lower factor loading for OP-AAv (0.45). Numerous studies have identified traffic emissions as a main source contributor to OP-AA (Pietrogrande et al. 2021; Calas et al. 2018; Campbell et al. 2021). The sensitivity of OP-AA to Mn alloy industry emissions is possibly due to Fe (split into factors 1 and 2) and to the high impact of this source in the area. In addition, the potential ability of Mn (III) to contribute to OP-AA cannot be ruled out and should be investigated, since a previous work demonstrated the presence of Mn(III) together with other oxidation states in the studied area (Hernández-Pellón et al. 2019).
Several investigations have studied the correlations between OP and PM species (Gao et al. 2020a) in which OP AA assay was frequently sensitive to metals (Calas et al. 2018; Perrone et al. 2019; Bates et al. 2019). In most of the studies, Cu and Fe were found strongly correlated with OP-AAv (Visentin et al. 2016; Fang et al. 2016; Calas et al. 2019; Perrone et al. 2019; Gao et al. 2020b; Gosselin and Zagury 2020). However, there is no consensus about the associations between OP-AA and the rest of the studied metal(loid)s. Moderate or low correlations with OP-AAv responses were obtained for Mn, Zn, Mo, V, Cd, Pb, As, Sb, and Ni (Szigeti et al. 2015; Moreno et al. 2017; Calas et al. 2018; Pietrogrande et al. 2018; Weber et al. 2021).
The different correlations between OP and chemical composition can be affected by many factors such as the solubility of the compounds, the chemical form, the element concentrations, and the OP measurement conditions (Moreno et al. 2017; Bates et al. 2019). Moreover, associations between some species and OP could be due to intercorrelations between chemical compounds rather than a significant causal relationship between OP assay and PM components (Charrier and Anastasio 2012; Borlaza et al. 2021). In particular, in the studied area, almost 60% of metal(loid) concentrations are sourced from the manganese alloy plant (Hernández-Pellón and Fernández-Olmo 2019). In fact, among the studied metal(loid)s, only Cu and Fe are known to be very reactive in the AA assay, at least at neutral pH (Buettner 1988; Ayres et al. 2008; DiStefano et al. 2009; Guo et al. 2020). Our results with chemical compounds suggest that Cu (II) is able to catalyze the AA oxidation also in acidic conditions at the concentration levels found for PM2.5 samples studied in this work. With respect to the other studied metal(loid)s, it is unclear whether they might contribute to the AA consumption. Iron (III) seems also to be active as catalysts for the oxidation of AA while Mn (II), Ni (II), and Zn (II) were found to be inactive (Khan and Martell 1967). However, Visentin et al. (2016) showed that Zn (II) and Ni (II) are reactive ions in OP-AA assay and Mn (II) has a very low AA activity. Martell (1982) suggested that in addition to Cu (II) and Fe (III), many other metal ions are potentially capable of undergoing redox reactions with ascorbate, catalyzing its oxidation. However, Khan and Martell (1967) hypothesized that the electron-transfer process from the ascorbate anion to the metal ion involves the reducibility of the metal cation. Unless the metal cation has a stable lower valence form, such as Cu (II/I) or Fe (III/II), it will not be expected to participate in the electron transfer mechanism and thus will not be active as catalysts in the AA oxidation. Additional research with more chemical compounds (e.g., Mn (III)) is needed to identify the active species in the AA assay, both at neutral and acidic pH.
Conclusions
Our results showed firstly that the AA depletion from three catalytic compounds (Cu salts and 1,4-NQ) in an acidic fluid (ALF) was much lower than in a neutral fluid (PBS1x). The lower AA consumption in ALF is partly explained by considering the lower amount of AH− in this medium. In addition, the AA depletion rate seems to depend on the sensitivity of each catalytic compound to the pH and composition of the fluid, although further investigations are needed to understand how PM components affect OP consumption in the OP-AA assay at both neutral and acidic pH.
The low OP-AA values found with the studied chemical species in ALF could partly explain the low values obtained in ALF from PM2.5 samples collected in an urban-industrial site, compared to the studies found in the literature, carried out at neutral pH conditions. Nevertheless, the sensitivity of the OP-AA assay in ALF is enough to quantify it (i.e., almost all PM2.5 OP-AA values were above the detection limit). In addition, significant positive correlations were obtained between OP-AAv and ALF-bioaccessible concentrations of most of the studied metal(loid)s. Copper and Fe showed the highest correlation with OP-AA, which is in agreement with the PCA results, where the highest loadings for the factor attributed to road traffic were obtained for OP-AA, Cu, and Fe; the high correlations of other metal(loid)s with OP-AA (Sb, As, Mn, Mo) could be due to intercorrelations between metal(loid)s. Further investigations are needed to study the role of other metal(loid)s besides Cu in the ascorbate oxidation under acidic conditions.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Arruti A, Fernández-Olmo I, Irabien A (2011) Impact of the global economic crisis on metal levels in particulate matter (PM) at an urban area in the Cantabria Region (Northern Spain). Environ Pollut 159(5):1129–1135. https://doi.org/10.1016/j.envpol.2011.02.008
Ayres JG, Borm P, Cassee FR, Castranova V, Donaldson K, Ghio A, Harrison RM, Hider R, Kelly F, Kooter IM, Marano F, Maynard RL, Mudway I, Nel A, Sioutas C, Smith S, Baeza-Squiban A, Cho A, Duggan S, Froines J (2008) Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential - a workshop report and consensus statement. Inhal Toxicol 20(1):75–99. https://doi.org/10.1080/08958370701665517
Bates JT, Fang T, Verma V, Zeng L, Weber RJ, Tolbert PE, Abrams JY, Sarnat SE, Klein M, Mulholland JA, Russell AG (2019) Review of acellular assays of ambient particulate matter oxidative potential: methods and relationships with composition, sources, and health effects. Environ Sci Technol 53(8):4003–4019. https://doi.org/10.1021/acs.est.8b03430
Borlaza LJS, Weber S, Jaffrezo JL, Houdier S, Slama R, Rieux C, Albinet A, Micallef S, Trébluchon C, Uzu G (2021) Disparities in particulate matter (PM10) origins and oxidative potential at a city scale (Grenoble, France) - Part 2: Sources of PM10 oxidative potential using multiple linear regression analysis and the predictive applicability of multilayer perceptron n. Atmos Chem Phys 21(12):9719–9739. https://doi.org/10.5194/acp-21-9719-2021
Buettner GR (1988) In the absence of catalytic metals ascorbate does not autoxidize at pH 7: ascorbate as a test for catalytic metals. J Biochem Biophys Methods 16(1):27–40. https://doi.org/10.1016/0165-022X(88)90100-5
Calas A, Uzu G, Besombes JL, Martins JMF, Redaelli M, Weber S, Charron A, Albinet A, Chevrier F, Brulfert G, Mesbah B, Favez O, Jaffrezo JL (2019) Seasonal variations and chemical predictors of oxidative potential (OP) of particulate matter (PM), for seven urban French sites. Atmos 10(11):1–20. https://doi.org/10.3390/atmos10110698
Calas A, Uzu G, Kelly FJ, Houdier S, Martins JMF, Thomas F, Molton F, Charron A, Dunster C, Oliete A, Jacob V, Besombes JL, Chevrier F, Jaffrezo JL (2018) Comparison between five acellular oxidative potential measurement assays performed with detailed chemistry on PM10 samples from the city of Chamonix (France). Atmos Chem Phys 18(11):7863–7875. https://doi.org/10.5194/acp-18-7863-2018
Calas A, Uzu G, Martins JMF, Voisin D, Spadini L, Lacroix T, Jaffrezo JL (2017) The importance of simulated lung fluid (SLF) extractions for a more relevant evaluation of the oxidative potential of particulate matter. Sci Rep 7(1):1–12. https://doi.org/10.1038/s41598-017-11979-3
Campbell SJ, Wolfer K, Utinger B, Westwood J, Zhang Z-H, Bukowiecki N, Steimer SS, Vu TV, Xu J, Straw N, Thomson S, Elzein A, Sun Y, Liu D, Li L, Fu P, Lewis AC, Harrison RM, Bloss WJ et al (2021) Atmospheric conditions and composition that influence PM2.5 oxidative potential in Beijing, China. Atmos Chem Phys 21(7):5549–5573. https://doi.org/10.5194/acp-21-5549-2021
Cervellati F, Benedusi M, Manarini F, Woodby B, Russo M, Valacchi G, Pietrogrande MC (2020) Proinflammatory properties and oxidative effects of atmospheric particle components in human keratinocytes. Chemosphere 240:124746. https://doi.org/10.1016/j.chemosphere.2019.124746
Charrier JG, Anastasio C (2012) On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: evidence for the importance of soluble transition metals. Atmos Chem Phys 12(19):9321–9333. https://doi.org/10.5194/acp-12-9321-2012
Cigánková H, Mikuška P, Hegrová J, Krajčovič J (2021) Comparison of oxidative potential of PM1 and PM2.5 urban aerosol and bioaccessibility of associated elements in three simulated lung fluids. Sci Total Environ 800. https://doi.org/10.1016/j.scitotenv.2021.149502
Clemente Á, Gil-Moltó J, Yubero E et al (2023) Sensitivity of PM10 oxidative potential to aerosol chemical composition at a Mediterranean urban site: ascorbic acid versus dithiothreitol measurements. Air Qual Atmos Health. https://doi.org/10.1007/s11869-023-01332-1
Colombo C, Monhemius AJ, Plant JA (2008) Platinum, palladium and rhodium release from vehicle exhaust catalysts and road dust exposed to simulated lung fluids. Ecotoxicol Environ Safety 71(3):722–730. https://doi.org/10.1016/j.ecoenv.2007.11.011
Distefano E, Eiguren-Fernandez A, Delfino RJ, Sioutas C, Froines JR, Cho AK (2009) Determination of metal-based hydroxyl radical generating capacity of ambient and diesel exhaust particles. Inhal Toxicol 21(9):731–738. https://doi.org/10.1080/08958370802491433
Du J, Cullen JJ, Buettner GR (2012) Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim Biophys Acta (BBA) – Rev Cancer 1826(2):443–457. https://doi.org/10.1016/j.bbcan.2012.06.003
Expósito A, Markiv B, Ruiz-Azcona L, Santibáñez M, Fernández-Olmo I (2021a) Understanding how methodological aspects affect the release of trace metal(loid)s from urban dust in inhalation bioaccessibility tests. Chemosphere 267. https://doi.org/10.1016/j.chemosphere.2020.129181
Expósito A, Markiv B, Ruiz-Azcona L, Santibáñez M, Fernández-Olmo I (2021b) Personal inhalation exposure to manganese and other trace metals in an environmentally exposed population: bioaccessibility in size-segregated particulate matter samples. Atmos Pollut Res 12(8). https://doi.org/10.1016/j.apr.2021.101123
Fang T, Verma V, Bates JT, Abrams J, Klein M, Strickland MJ, Sarnat SE, Chang HH, Mulholland JA, Tolbert PE, Russell AG, Weber RJ (2016) Oxidative potential of ambient water-soluble PM2.5 in the southeastern United States: Contrasts in sources and health associations between ascorbic acid (AA) and dithiothreitol (DTT) assays. Atmos Chem Phys 16(6):3865–3879. https://doi.org/10.5194/acp-16-3865-2016
Frezzini MA, de Francesco N, Massimi L, Canepari S (2022) Effects of operating conditions on PM oxidative potential assays. Atmos Environ 268:118802. https://doi.org/10.1016/j.atmosenv.2021.118802
Gao D, Ripley S, Weichenthal S, Godri Pollitt KJ (2020a) Ambient particulate matter oxidative potential: chemical determinants, associated health effects, and strategies for risk management. Free Radic Biol Med 151:7–25. https://doi.org/10.1016/j.freeradbiomed.2020.04.028
Gao D, Godri Pollitt KJ, Mulholland JA, Russell AG, Weber RJ (2020b) Characterization and comparison of PM2.5 oxidative potential assessed by two acellular assays. Atmos Chem Phys 20(9):5197–5210. https://doi.org/10.5194/acp-20-5197-2020
Ghio AJ, Carraway MS, Madden MC (2012) Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J Toxicol Environ Health - Part B: Critical Reviews 15(1):1–21. https://doi.org/10.1080/10937404.2012.632359
Gosselin M, Zagury GJ (2020) Metal(loid)s inhalation bioaccessibility and oxidative potential of particulate matter from chromated copper arsenate (CCA)-contaminated soils. Chemosphere 238. https://doi.org/10.1016/j.chemosphere.2019.124557
Guo H, Fu H, Jin L, Huang S, Li X (2020) Quantification of synergistic, additive and antagonistic effects of aerosol components on total oxidative potential. Chemosphere 252:126573. https://doi.org/10.1016/j.chemosphere.2020.126573
Guo H-b, Li M, Lyu Y, Cheng T-t, Xv J-j, Li X (2019) Size-resolved particle oxidative potential in the office, laboratory, and home: evidence for the importance of water-soluble transition metals. Environ Pollut 246:704–709. https://doi.org/10.1016/j.envpol.2018.12.094
Hayakawa K, Minami S, Nakamura S (1973) Kinetics of oxidation of ascorbic acid by the copper(ii) ion an acetate buffer solution. Bull Chem Soc Japn 26:2788–2791
He RW, Shirmohammadi F, Gerlofs-Nijland ME, Sioutas C, Cassee FR (2018) Pro-inflammatory responses to PM 0.25 from airport and urban traffic emissions. Sci Total Environ 640–641:997–1003. https://doi.org/10.1016/j.scitotenv.2018.05.382
Hedayat F, Stevanovic S, Miljevic B, Bottle S, Ristovski ZD (2015) Review-evaluating the molecular assays for measuring the oxidative potential of particulate matter. Chem Ind Chem Eng Q 21(1–2):201–210. https://doi.org/10.2298/CICEQ140228031H
Hernández-Pellón A, Fernández-Olmo I (2019) Using multi-site data to apportion PM-bound metal(loid)s: Impact of a manganese alloy plant in an urban area. Sci Total Environ 651:1476–1488. https://doi.org/10.1016/j.scitotenv.2018.09.261
Hernández-Pellón A, Mazón P, Fernández-Olmo I (2019) Quantification of manganese species in particulate matter collected in an urban area nearby a manganese alloy plant. Atmos Environ 205:46–51. https://doi.org/10.1016/j.atmosenv.2019.02.040
Hernández-Pellón A, Nischkauer W, Limbeck A, Fernández-Olmo I (2018) Metal(loid) bioaccessibility and inhalation risk assessment: a comparison between an urban and an industrial area. Environ Res 165:140–149. https://doi.org/10.1016/j.envres.2018.04.014
Innes E, Yiu HHP, McLean P, Brown W, Boyles M (2021) Simulated biological fluids–a systematic review of their biological relevance and use in relation to inhalation toxicology of particles and fibres. Crit Rev Toxicol 51(3):217–248. https://doi.org/10.1080/10408444.2021.1903386
Kelly FJ, Fussell JC (2012) Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos Environ 60:504–526. https://doi.org/10.1016/j.atmosenv.2012.06.039
Khan MMT, Martell AE (1967) Metal ion and metal chelate catalyzed oxidation of ascorbic acid by molecular oxygen. II. Cupric and Ferric Chelate Catalyzed Oxidation. J American Chem Society 89(26):7104–7111. https://doi.org/10.1021/ja01002a046
Khoshkam Z, Habibi-Rezaei M, Hassanvand MS, Aftabi Y, Seyedrezazadeh E, Amiri-Sadeghan A, Zarredar H, Roshangar L, Gholampour A, Moosavi-Movahedi AA (2022) The oxidative and neurotoxic potentials of the ambient PM2.5 extracts: the efficient multi-solvent extraction method. Sci Total Environ 810:152291. https://doi.org/10.1016/j.scitotenv.2021.152291
Khoshnamvand N, Nodehi RN, Hassanvand MS et al (2023) Comparison between oxidative potentials measured of water-soluble components in ambient air PM1 and PM2.5 of Tehran, Iran. Air Qual Atmos Health. https://doi.org/10.1007/s11869-023-01343-y
Kim KH, Kabir E, Kabir S (2015) A review on the human health impact of airborne particulate matter. Environ Int 74:136–143. https://doi.org/10.1016/j.envint.2014.10.005
Li Q, Wyatt A, Kamens RM (2009) Oxidant generation and toxicity enhancement of aged-diesel exhaust. Atmos Environ 43(5):1037–1042. https://doi.org/10.1016/j.atmosenv.2008.11.018
Li Z, Nie D, Chen M, Ge P, Liu Z, Ma X, Ge X, Gu R (2021) Seasonal variation of oxidative potential of water-soluble components in PM2.5 and PM1 in the Yangtze River Delta, China. Air Quality Atmos Health 14(11):1825–1836. https://doi.org/10.1007/s11869-021-01056-0
Macartney DH, Sutin N (1983) The Oxidation of Ascorbic Acid by Tris(2,2’-bipyridine) Complexes of Osmium(III), Ruthenium(II1) and Nickel(II1) in Aqueous Media: Applications of the Marcus Cross-Relation. Inorg Chim Acta 74:221–228. https://doi.org/10.1016/j.molliq.2020.113154
Mapson LW (1945) Influence of halides on the oxidation of ascorbic acid; action of Cl- on the cupric-cuprous system. Biochem J 39(3):228–236. https://doi.org/10.1042/bj0390228
Markiv B, Ruiz-Azcona L, Expósito A, Santibáñez M, Fernández-Olmo I (2022) Short- and long-term exposure to trace metal(loid)s from the production of ferromanganese alloys by personal sampling and biomarkers. Environ Geochem Health. https://doi.org/10.1007/s10653-022-01218-8
Martell AE (1982) Chelates of ascorbic acid. In: Ascorbic acid: chemistry, metabolism, and uses, pp 153–178. https://doi.org/10.1021/ba-1982-0200.ch007
Møller P, Danielsen PH, Karottki DG, Jantzen K, Roursgaard M, Klingberg H, Jensen DM, Christophersen DV, Hemmingsen JG, Cao Y, Loft S (2014) Oxidative stress and inflammation generated DNA damage by exposure to air pollution particles. Mut Res Rev in Mutat Res 762:133–166. https://doi.org/10.1016/j.mrrev.2014.09.001
Moreno T, Kelly FJ, Dunster C, Oliete A, Martins V, Reche C, Minguillón MC, Amato F, Capdevila M, de Miguel E, Querol X (2017) Oxidative potential of subway PM2.5. Atmos Environ 148:230–238. https://doi.org/10.1016/j.atmosenv.2016.10.045
Moufarrej L, Courcot D, Ledoux F (2020) Assessment of the PM2.5 oxidative potential in a coastal industrial city in Northern France: relationships with chemical composition, local emissions and long range sources. Sci Total Environ 748. https://doi.org/10.1016/j.scitotenv.2020.141448
Mukhtar A, Limbeck A (2013) Recent developments in assessment of bio-accessible trace metal fractions in airborne particulate matter: A review. Anal Chim Acta 774:11–25. https://doi.org/10.1016/j.aca.2013.02.008
Murekhina AE, Yarullin DN, Sovina MA, Kitaev PA, Gamov GA (2022) Copper (II)-catalyzed oxidation of ascorbic acid: ionic strength effect and analytical use in aqueous solution. Inorganics 10(7):1–12. https://doi.org/10.3390/inorganics10070102
Otero-Pregigueiro D, Fernández-Olmo I (2018) Use of CALPUFF to predict airborne Mn levels at schools in an urban area impacted by a nearby manganese alloy plant. Environ Int 119:455–465. https://doi.org/10.1016/j.envint.2018.07.005
Øvrevik J (2019) Oxidative potential versus biological effects: a review on the relevance of cell-free/abiotic assays as predictors of toxicity from airborne particulate matter. Int J Mol Sci 20(19). https://doi.org/10.3390/ijms20194772
Pant P, Harrison RM (2013) Estimation of the contribution of road traffic emissions to particulate matter concentrations from field measurements: a review. Atmos Environ 77:78–97. https://doi.org/10.1016/j.atmosenv.2013.04.028
Park J, Park EH, Schauer JJ, Yi SM, Heo J (2018) Reactive oxygen species (ROS) activity of ambient fine particles (PM2.5) measured in Seoul, Korea. Environ Int 117:276–283. https://doi.org/10.1016/j.envint.2018.05.018
Pelfrêne A, Cave MR, Wragg J, Douay F (2017) In vitro investigations of human bioaccessibility from reference materials using simulated lung fluids. Int J Environ Res Public Health 14(2):1–15. https://doi.org/10.3390/ijerph14020112
Perrone MR, Bertoli I, Romano S, Russo M, Rispoli G, Pietrogrande MC (2019) PM2.5 and PM10 oxidative potential at a Central Mediterranean Site: contrasts between dithiothreitol- and ascorbic acid-measured values in relation with particle size and chemical composition. Atmos Environ 210:143–155. https://doi.org/10.1016/j.atmosenv.2019.04.047
Pietrogrande MC, Bacco D, Trentini A, Russo M (2021) Effect of filter extraction solvents on the measurement of the oxidative potential of airborne PM2.5. Environ Sci Pollut Res 28(23):29551–29563. https://doi.org/10.1007/s11356-021-12604-7
Pietrogrande MC, Bertoli I, Manarini F, Russo M (2019) Ascorbate assay as a measure of oxidative potential for ambient particles: evidence for the importance of cell-free surrogate lung fluid composition. Atmos Environ 211:103–112. https://doi.org/10.1016/j.atmosenv.2019.05.012
Pietrogrande MC, Dalpiaz C, Dell’Anna R, Lazzeri P, Manarini F, Visentin M, Tonidandel G (2018) Chemical composition and oxidative potential of atmospheric coarse particles at an industrial and urban background site in the alpine region of northern Italy. Atmos Environ 191:340–350. https://doi.org/10.1016/j.atmosenv.2018.08.022
Pietrogrande MC, Demaria G, Colombi C, Cuccia E, Dal Santo U (2022b) Seasonal and spatial variations of PM10 and PM2.5 oxidative potential in five urban and rural sites across Lombardia Region, Italy. Int J Environ Res Public Health 19(13). https://doi.org/10.3390/ijerph19137778
Pietrogrande MC, Romanato L, Russo M (2022a) Synergistic and antagonistic effects of aerosol components on its oxidative potential as predictor of particle toxicity. Toxics 10(4). https://doi.org/10.3390/toxics10040196
Pope CA, Coleman N, Pond ZA, Burnett RT (2020) Fine particulate air pollution and human mortality: 25+ years of cohort studies. Environ Res 183:108924. https://doi.org/10.1016/j.envres.2019.108924
Ristovski ZD, Miljevic B, Surawski NC, Morawska L, Fong KM, Goh F, Yang IA (2012) Respiratory health effects of diesel particulate matter. Respirology 17(2):201–212. https://doi.org/10.1111/j.1440-1843.2011.02109.x
Roth JA (2006) Homeostatic and toxic mechanisms regulating manganese uptake, retention, and elimination. Bio Res 39:45–57. https://doi.org/10.4067/S0716-97602006000100006
Ruiz S, Fernández-Olmo I, Irabien Á (2014) Discussion on graphical methods to identify point sources from wind and particulate matter-bound metal data. Urban Clim 10:671–681. https://doi.org/10.1016/j.uclim.2013.11.001
Şahbaz F, Somer G (1993) The effect of citrate anions on the kinetics of cupric ion-catalysed oxidation of ascorbic acid. Food Chem 47(4):345–349. https://doi.org/10.1016/0308-8146(93)90175-F
Sauvain JJ, Deslarzes S, Storti F, Riediker M (2014) Oxidative potential of particles in different occupational environments: a pilot study. Ann Occup Hyg 59(7):882–894. https://doi.org/10.1093/annhyg/mev024
Schiavo B, Meza-Figueroa D, Vizuete-Jaramillo E, Robles-Morua A, Angulo-Molina A, Reyes-Castro PA, Inguaggiato C, Gonzalez-Grijalva B, Pedroza-Montero M (2022) Oxidative potential of metal-polluted urban dust as a potential environmental stressor for chronic diseases. Environ Geochem Health. https://doi.org/10.1007/s10653-022-01403-9
Seinfeld JH, Pandis SN (2016) Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 2nd edn. John Wiley & Sons, Hoboken, New Jersey
Shahpoury P, Harner T, Lammel G, Lelieveld S, Tong H, Wilson J (2019) Development of an antioxidant assay to study oxidative potential of airborne particulate matter. Atmos Meas Tech 12(12):6529–6539. https://doi.org/10.5194/amt-12-6529-2019
Shen J, Griffiths PT, Campbell SJ, Utinger B, Kalberer M, Paulson SE (2021) Ascorbate oxidation by iron, copper and reactive oxygen species: review, model development, and derivation of key rate constants. Sci Rep 11(1):1–14. https://doi.org/10.1038/s41598-021-86477-8
Shiraiwa M, Ueda K, Pozzer A, Lammel G, Kampf CJ, Fushimi A, Enami S, Arangio AM, Fröhlich-Nowoisky J, Fujitani Y, Furuyama A, Lakey PSJ, Lelieveld J, Lucas K, Morino Y, Pöschl U, Takahama S, Takami A, Tong H et al (2017) Aerosol health effects from molecular to global scales. Environ Sci Technol 51(23):13545–13567. https://doi.org/10.1021/acs.est.7b04417
Song M, Oh S, Park C, Bae M (2021) Review: analytical procedure for dithiothreitol-based. Asian J Atmos Environ 15(1):1–13. https://doi.org/10.5572/ajae.2021.015
Szigeti T, Óvári M, Dunster C, Kelly FJ, Lucarelli F, Záray G (2015) Changes in chemical composition and oxidative potential of urban PM2.5 between 2010 and 2013 in Hungary. Sci Total Environ 518–519:534–544. https://doi.org/10.1016/j.scitotenv.2015.03.025
Tian Y, Xiang X, Juan J, Sun K, Song J, Cao Y, Hu Y (2017) Fine particulate air pollution and hospital visits for asthma in Beijing, China. Environ Pollut 230:227–233. https://doi.org/10.1016/j.envpol.2017.06.029
Van den Heuvel R, den Hond E, Govarts E, Colles A, Koppen G, Staelens J, Mampaey M, Janssen N, Schoeters G (2016) Identification of PM10 characteristics involved in cellular responses in human bronchial epithelial cells (Beas-2B). Environ Res 149:48–56. https://doi.org/10.1016/j.envres.2016.04.029
Visentin M, Pagnoni A, Sarti E, Pietrogrande MC (2016) Urban PM2.5 oxidative potential: importance of chemical species and comparison of two spectrophotometric cell-free assays. Environ Pollut 219:72–79. https://doi.org/10.1016/j.envpol.2016.09.047
Wallenborn JG, McGee JK, Schladweiler MC, Ledbetter AD, Kodavanti UP (2007) Systemic translocation of particulate matter-associated metals following a single intratracheal instillation in rats. Toxicol Sci 98(1):231–239. https://doi.org/10.1093/toxsci/kfm088
Weber S, Uzu G, Favez O, Borlaza LJS, Calas A, Salameh D, Chevrier F, Allard J, Besombes J-L, Albinet A, Pontet S, Mesbah B, Gille G, Zhang S, Pallares C, Leoz-Garziandia E, Jaffrezo J-L (2021) Source apportionment of atmospheric PM10 oxidative potential: synthesis of 15 year-round urban datasets in France. Atmos Chem Phys 21(14):11353–11378. https://doi.org/10.5194/acp-21-11353-2021
Wei J, Fang T, Lakey PSJ, Shiraiwa M (2021) Iron-facilitated organic radical formation from secondary organic aerosols in surrogate lung fluid. Environ Sci Technol. https://doi.org/10.1021/acs.est.1c04334
Weichenthal SA, Pollitt KG, Villeneuve PJ (2013) PM2.5, oxidant defence and cardiorespiratory health: a review. Environ Health 12:40. https://doi.org/10.1186/1476-069X-12-40
Weissberger A, LuValle JE, Thomas DS (1943) The autoxidation of ascorbic acid. J Am Chem Soc 65(6):1212–1217
World Health Organization (WHO). Regional office for Europe. (2000). Air Quality Guidelines for Europe. World Health Organization Regional Publications, European Series, No. 91. https://apps.who.int/iris/handle/10665/107335. Accessed 19 Sept 2023
Wu N, Lyu Y, Lu B, Cai D, Meng X, Li X (2022) Oxidative potential induced by metal-organic interaction from PM2.5 in simulated biological fluids. Sci Total Environ 848:157768. https://doi.org/10.1016/j.scitotenv.2022.157768
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was supported by the Spanish Ministry of Science and Innovation (Project PID2020-114787RB-I00, funded by MCIN/AEI/10.13039/501100011033 and “ERDF A way of making Europe”). Bohdana Markiv also thanks the MICIU for her predoctoral contract (PRE2018-085152, financed together by MCIN/AEI/10.13039/501100011033 and “ESF Investing in your future”).
Author information
Authors and Affiliations
Contributions
A. Expósito: investigation, writing—original draft preparation, and formal analysis; B. Markiv: investigation; M. Santibáñez: supervision and funding acquisition; M. Fadel: investigation, writing—review and editing; F. Ledoux: writing—review and editing—and supervision; D. Courcot: writing—review and editing—and supervision; I. Fernández-Olmo: conceptualization, methodology, writing—review and editing, supervision, and funding acquisition.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
There is no need for consent approval.
Consent for publication
The manuscript did not contain any individual person data in any form.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 31 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Expósito, A., Markiv, B., Santibáñez, M. et al. Ascorbate oxidation driven by PM2.5-bound metal(loid)s extracted in an acidic simulated lung fluid in relation to their bioaccessibility. Air Qual Atmos Health 17, 177–189 (2024). https://doi.org/10.1007/s11869-023-01436-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-023-01436-8