Abstract
Indoor and outdoor PM2.5 samples were collected in three residential areas near a ferromanganese smelter using GilAir300 plus at 2.75 L/min, and the elemental composition was analysed using inductively coupled plasma-mass spectroscopy. A health risk assessment was conducted to determine the probability of developing carcinogenic and non-carcinogenic effects for four age groups. The hazard quotient (HQ) for manganese was >1 both indoors and outdoors for the four age groups in all residential areas, indicating a risk of developing non-carcinogenic health effects. The HQs of Cr (VI) displayed a similar trend for all age groups; it was >1 in all residential areas except for outdoor environments at New Sicelo. The highest HQ (25.6) was found indoors at Old Sicelo for the 21–35 age group whereas the minimum (8.3) was found indoors at Noldick for the 36–65+ age group. When using the overall concentrations, the HQ was >1 only for Mn and the highest values were recorded at Noldick. The cancer risks for chromium (VI), cobalt, and cadmium were above the upper limit of 1 × 10−4 and the lower limit of 1 × 10−6 when considering indoor and outdoor concentrations. When considering the overall concentrations, the cancer risk for cobalt was >1 × 10−6 and that of chromium (VI) was >1 × 10−4. Urgent intervention is required, particularly given the negative health effects associated with Mn exposure.
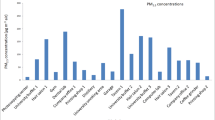
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Air pollution is a significant public health and environmental problem globally, particularly in industrialised urban areas in low-to-middle-income countries (Roy 2016), where it is associated with adverse health effects that have significant socio-economic implications (Mannucci and Franchini 2017). High-temperature sources such as ferromanganese smelters are significant anthropogenic sources (Markiv et al. 2022) that release particulate matter (PM) with different physicochemical properties (Hernández-Pellón et al. 2017) that contribute to air pollution, mostly in urban areas (World Health Organization 2016). PM can adsorb inorganic and organic metal(loid)s, subsequently becoming a repository and a vehicle (Lovaković et al. 2022). Ferromanganese smelters tend to release smaller particles (aerodynamic diameter < 2.5 μm) (Fernández-Camacho et al. 2012) that can remain suspended longer in the atmosphere and can be transported long distances from the source (Mansha et al. 2012), subsequently causing poor indoor and outdoor air quality in nearby and downwind areas (Hernández-Pellón and Fernández-Olmo 2019a).
Residential areas near and downwind of ferromanganese smelters tend to have higher concentrations of PM-bound metal(loid)s than upwind and farther areas (Haynes et al. 2012; Hernández-Pellón and Fernández-Olmo 2016). For example, Boudissa et al. (2006) found that the mean concentration of respirable Mn in an area downwind of a non-active Mn alloy production plant was 26-fold higher than that in upwind areas. Racette et al. (2021a) recently found that the 2-year PM2.5-bound Mn concentration was 203 ng/m3 for a residential area downwind of a ferromanganese smelter and 10 ng/m3 in a residential area upwind. Despite these findings, most studies assessing exposure to airborne Mn have been conducted in occupational settings where exposure is managed through reduction interventions, employee training, and the use of personal protective equipment (Davourie et al. 2017). However, in residential areas, where airborne Mn can contribute to poor indoor and outdoor air quality, little is being done to manage the risk of exposure. Furthermore, exposure to Mn-bearing particles in occupational settings is characterised by intermittently high concentrations, whereas environmental exposure consists of low concentrations for a prolonged period through multiple exposure routes (Solís-Vivanco et al. 2009).
The reluctance to conduct studies in residential areas is due to the anticipated low concentrations (Sly et al. 2016). However, it has been shown that some metal(loid)s can induce adverse health effects even at low concentrations (Lovaković et al. 2022). The health effects of exposure to indoor PM2.5-bound metal(loid)s have been reported in susceptible groups, such as the elderly (Abdel-Salam 2022), children (Lovaković et al. 2022), pregnant women, and immunocompromised individuals who spend most of their time indoors (Delgado-Saborit 2019). For example, Kim and Kang (1997) found greater deposition of particles in subjects with obstructive lung disorders than in healthy subjects.
Evidence suggests that the toxicity of PM is influenced by the number concentration (Greig 2000), size (Schwartz et al. 2002), elemental composition (Goix et al. 2014), solubility (Kelly and Fussell 2012), and oxidation state (Bollati et al. 2010), whereas the health outcomes and their severity depend on the route of entry (Morawska et al. 2013), exposure duration, concentration (Hammer et al. 2021), and individual characteristics (Kim et al. 2015) and activities of the individual (Löndahl et al. 2007). Smaller particles (<2.5 μm) can deposit and penetrate deeper alveoli regions (Geiser et al. 2005), bypass the olfactory system, translocate directly to the brain (Tsuda et al. 2013) through the blood-cerebrospinal fluid barrier (Bornhorst et al. 2012), enter the blood circulation system (Fiordelisi et al. 2017), and translocate to vital organs such as the heart, liver, and spleen, causing systemic effects (Ali et al. 2022). Although the deposition efficiency of smaller particles is 50% (Hinds 1999), they are not easily cleared by macrophages; hence, they can be retained for up to 7 days (Mbazima 2022).
Several health risk assessment (HRA) studies (Hernández-Pellón et al. 2017, 2018; Expósito et al. 2021) have reported a higher risk of developing carcinogenic and non-carcinogenic adverse health outcomes in populations living near ferromanganese smelters. However, most of these studies used data obtained from fixed monitoring stations located further from receptors; subsequently, different populations in different microenvironments were assigned the same concentration of pollutants (Monn 2002). Brokamp et al. (2015) argued that data obtained from distances away from receptors are not a true representation of exposure in the near field. The present paradigm of exposure assessments calls for collecting data at the receptor level to obtain representative results that are not misleading (Brokamp et al. 2015). Moreover, most HRA studies only focused on outdoor air pollutants and neglected the indoor environment; however, exposure to PM2.5-bound metal(loid)s frequently occurs in indoor environments where many people spend most of their time (Morawska et al. 2017). Therefore, a HRA study that considers exposure to PM2.5-bound metals in indoor and outdoor environments is essential to avoid bias and misclassification.
This study determined the probability of developing carcinogenic and non-carcinogenic health effects among four age groups through the inhalation route based on the concentrations of indoor and outdoor PM2.5-bound metal(loid)s and overall concentrations in three residential areas downwind of a ferromanganese smelter in Meyerton, Republic of South Africa (RSA). This is the first study conducted in residential areas near a ferromanganese smelter in RSA and can be used as a baseline of what Meyerton residents are exposed to and the potential of developing adverse health effects. The findings of this study can help to determine the types of interventions needed and where they can be implemented to effectively protect public health.
Methods and materials
Study area
Meyerton is a town located 15 km north of Vereeniging, Gauteng Province, with GPS coordinates 26.5854° S and 28.0069° E. Meyerton has an area of 180.24 km2 and a population size of 55,283 people consisting of 28,041 (50.72%) males and 27,242 (49.28%) females (Statistics South Africa 2011). According to the most recent census, 22.7% of the population is between the ages of 0 and 14, 70% are between 15 and 64, and 7% are 65 years and above (Statistics South Africa 2011). On average, Meyerton receives an average rainfall of 34.4 mm, wind speed of 6 km/h, humidity of 61%, and temperature of 17 °C. Approximately 77.5% of the households in Meyerton are formal dwellings, while the remaining are informal settlements made mainly of corrugated iron and boards (Statistics South Africa 2011).
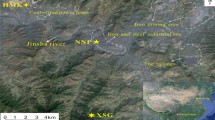
At the boundaries of Meyerton, a FeMn smelter is located upwind of three residential areas: Old Sicelo, New Sicelo, and Noldick. New Sicelo is closest to the FeMn smelter (~1.4 km), followed by Noldick (~1.5 km) and Old Sicelo (~3.5 km) (Fig. 1). The ferromanganese smelter has been operational in the Meyerton area since 1951 and is one of the largest Mn smelters globally that produces high-carbon-FeMn (Creamer 2013). FeMn is an important additive ingredient in the steelmaking industry because it provides distinctive physical characteristics, such as strength and flexibility (Pearson and Greenway 2005). In 2013, the ferromanganese smelter was installed with an 81 MVA furnace that can produce 120,000 tonnes of HCFeMn per year (Creamer 2013). According to Steyn (2018), the ferromanganese smelter can process more than 1 million tonnes of product annually.
Sampling
The sampling details have been described in detail by Mbazima et al. (2021). Briefly, a hardcopy map of the study areas was obtained from the Midvaal Local Municipality Office, and grids were drawn on the map to divide the residential areas. Two representative households were selected for the study. Specifically, the selected households were those of people who gave hair and nail samples and went for magnetic resonance imaging scans under the broad study titled, “Manganese exposure in an African community”. Indoor and outdoor PM2.5 samples were collected synchronously from selected households using two identical GilAir300 plus pumps (Sensidyne, St. Petersburg, FL, USA) at a flow rate of 2.75 L/min. The pumps were connected to a ~1.5-m Teflon tube joined to a 37-mm cassette housing polycarbonate membrane filter (Zefon, Ocala, FL, USA) with a diameter of 37 mm and a pore size of 0.08 m. The cassette was joined with a 37-mm PM2.5 cyclone (SKC, Inc., PA, USA) that used centrifugal force to separate the coarse and fine particles. Indoor PM2.5 was measured at a height of 1.5 m, 1.2 m away from walls and openings, and 1 m away from indoor sources such as cooking burners. Outdoor PM2.5 was measured at varying heights because of the different structures. For example, some structures were shacks made of corrugated iron and boards and some were made of brick and mortar. The indoor and outdoor PM2.5, measured for 24 h over 7 days from September to November 2019, yielded a total of 60 samples (30 indoor and 30 outdoor). Table 1 shows the details of the sampling campaign and the meteorological conditions [Meyerton, ZA Weather in January 2019 (tcktcktck.org)] during the sampling period.
Gravimetric and elemental analysis
An electronic microbalance scale (Sartorius AG, Göttingen, Germany) with a sensitivity of 0.001 mg was used to weigh the filters before and after the sampling. The filters were weighed thrice, and the average mass was calculated. The concentration (μg/m3) was calculated by dividing the final corrected mass (μg) by volume (m3). PM2.5-bound metal(loid)s were analysed using a Perkin Elmer NexION 300 ICP-MS (Perkin Elmer, Waltham, MA, USA) at the University of Johannesburg Spectrum Laboratory. Manganese (Mn), magnesium (Mg), silicon (Si), potassium (K), lead (Pb), vanadium (V), chromium (Cr), iron (Fe), cobalt (Co), nickel (Ni), cadmium (Cd), copper (Cu), and zinc (Zn) were selected for the ICP-MS analysis. The filters were folded and placed within cleaned microwave digestion vessels. Each vessel received 9 mL of super-pure nitric acid (HNO3) and 1 mL of super-pure hydrogen peroxide (H2O2), which were sealed and placed in a microwave on Mars 6 (Mars CEM, Matthews, NC, USA). Each digested filter was filtered with a 0.45-m syringe and 10 mL of super-pure water.
Statistical analysis
Furthermore, an analysis of variance (ANOVA) single factor with no replication was performed to determine whether there was a difference between the PM2.5-bound metal(loid) concentrations both indoors and outdoors across the three residential areas. Regression analysis was also performed to determine the relationship between the indoor and outdoor concentrations. All statistical analyses were performed using Microsoft Excel version 2019 (Microsoft Corporation, Redmond, WA, USA), and the alpha level was set at 0.05 and a p value < 0.05, indicating a statistically significant difference or relationship.
Health risk assessment
A HRA is a systematic process that comprises hazard identification, exposure assessment, dose-response assessment, risk characterisation, and risk management (Neris et al. 2019). It is also an effective probabilistic tool used to assess the risk of adverse health effects from exposure to environmental contaminants (Neris et al. 2019). The purpose of a HRA is to quantitatively link hazard(s) with health risks to the exposed population, thereby providing a platform for risk management (Morawska et al. 2013). This HRA was conducted for carcinogenic and non-carcinogenic PM2.5-bound metal(loid)s using the United States Environmental Protection Agency (USEPA) guidelines (United States Environmental Protection Agency 2009). Data from the ICP-MS analysis were transferred to a 2019 version of the Microsoft Excel spreadsheet, where HRA was conducted.
Hazard identification
Based on ICP-MS analysis, metal(loid)s with different toxicities were detected in PM2.5, collected in indoor and outdoor environments. Some metal(loid)s are carcinogens, whereas others are non-carcinogens. Cd, Cr (VI), Co, and Ni are classified as class 1 carcinogens, whereas Pb is classified as a class 2B carcinogen by the International Council on Cancer Research (Straif et al. 2009). Si, Fe, Mg, Mn, Na, Zn, Cu, and V are classified as non-carcinogenic, and their excessive concentrations can cause adverse health effects.
Exposure assessment
In exposure assessment, the magnitude, duration, frequency of exposure, and occurrence of adverse health outcomes for a specific subpopulation are estimated under different exposure scenarios (United States Environmental Protection Agency 2000). Because the inhalation route is regarded as the most common and damaging route of entry relative to ingestion and dermal exposure, the HRA was conducted using the inhalation route based on supplemental guidance for inhalation risk assessment (part F) (United States Environmental Protection Agency 2009). Exposure assessment was performed using exposure scenarios, and it was assumed that the Meyerton population spent 80% of their time indoors and 20% outdoors.
Exposure scenario one comprised the age group between 1 and 5 years, which spent most of their time indoors at home or nursery school. It was estimated that they spend approximately 6 h at the nursery school, meaning they spend 18 h at home. Exposure scenario two comprised the age group between 6 and 20 years, which is still at the basic and high school education levels. Based on the South African 2020 school calendar, it was estimated that they spend 5 h at school and 2 h travelling to and from school, implying that they spend approximately 17 h at home.
Exposure scenario three comprised an age group of 21–35 years, otherwise known as adults, students, and the working class. It was calculated that they worked or attended school for 8 h a day. They spend a total of 3 h travelling to and from work or school. Exposure scenario four comprised the age group of 36–65+ years, which included the elderly. It was estimated that they spend most of their time indoors; therefore, it was calculated that they spend approximately 19.2 h indoors and 4.8 h outdoors. The calculated time that the four age groups in Meyerton spend indoors and outdoors is shown in Table 2.
The indoor and outdoor exposure concentrations (ECi/o) were calculated using Eq. (1), where CA is the concentration (μg/m3) of a given PM2.5-bound metal(loid), IR is the inhalation rate (m3/day), ET is the exposure time (h/day), EF is the exposure frequency (days/year), ED is the exposure duration (years), ATn is the averaging time (years/day/h), and TWACA is the time-weighted average concentration. For non-carcinogenic health effects, ATn = (ED × 365 days × 24 h) and for carcinogenic health effects, ATn = (62 years × 365 days × 24 h), where 62 years is the life expectancy in RSA (Statistics South Africa 2021).
To calculate the overall exposure concentration (ECoverall), the time-weighted average concentration (TWACA) was first calculated using Eq. (2), where Ci is the indoor concentration of a given metal(loid), ti is the time spent indoors for a specific age group, Co is the outdoor concentration of a given metal(loid), and to is the time spent outdoors for a specific age group.
Thereafter, the TWACA was used to calculate the ECoverall using Eq. (3).
Risk characterisation
Risk characterisation integrates toxicity and exposure assessment data to determine the probability, nature, and severity of potential adverse health outcomes which are important for decision-making (United States Environmental Protection Agency 2000). Reference toxicity parameters were obtained from the Environmental Protection Agency Integrated Risk Assessment System database (https://www.epa.gov/iris). Table 3 shows the reference concentration (RfC) and inhalation unit risk (IUR) values used in the health risk assessment of indoor and outdoor PM2.5-bound metal(loid)s.
To determine the risk of developing non-carcinogenic adverse health outcomes due to inhalation of PM2.5-bound metal(loid)s, the hazard quotient (HQ) and hazard index (HI) were calculated using Eqs. (4) and (5), respectively. The risk of developing carcinogenic health effects (CR) was calculated using Eq. (4), assuming a linear dose relationship at low doses.
where EC is the exposure concentration (μg/m3) calculated using Eq. (1), RfC is the reference concentration for a given metal(loid) obtained from the USEPA IRIS system, and IUR is the inhalation unit risk obtained from the USEPA. A HQ greater than one (>1) indicates that the exposed population is at risk of developing non-carcinogenic adverse health effects whereas a HQ less than one (<1) indicates less risk. Cancer risk is the probability of an individual developing any type of cancer from lifetime exposure to carcinogenic chemicals. The cancer risk is represented by an acceptable number of cancer cases in a population and the widely used scales of risks are 1 in 1,000,000 (1 × 10−6), 1 in 100,000 (1 × 10−5), and 1 in 10,000 (1 × 10−4) (Castro et al. 2020). According to the World Health Organization (WHO) threshold, lifetime cancer risk values between 1 × 10−6 and 1 × 10−5 are categorised as acceptable, while for risk values exceeding 1 × 10−4 (Demirel et al. 2014), action is required (Legay et al. 2011). The HI is exposure to multiple or a mixture of chemicals through the same route of entry. A HI > 1 also indicates a risk of developing non-carcinogenic health effects. Exposure to a mixture of metals may cause additive or synergistic health effects that are more severe than those of a single metal(loid) (Calderón et al. 2003).
Results
Indoor and outdoor PM2.5 elemental composition
The concentrations of PM2.5-bound metal(loid)s in the indoor and outdoor environments of the three residential areas are shown in Table 4. All PM2.5-bound-metal(loid)s analysed using ICP-MS were detected both indoors and outdoors, except for K (<10.67) and Cd (<0.033), which were below the limit of quantification (LoQ). The LoQ values of K and Cd were divided by the square root of two (LoQ/\(\sqrt{2}\)) to obtain the adjusted concentrations (Hornung and Reed 1990). The dominant metal(loid)s in both the indoor and outdoor environments were in the order of K > Fe > Si > Mn > Mg. As shown in Table 4, the indoor airborne PM2.5-bound Mn concentrations were highest in Noldick, followed by Old Sicelo and New Sicelo.
Relationship between indoor and outdoor PM2.5-bound metal(loid)s
There was a difference between the concentrations of indoor and PM2.5-bound-metal(loid)s in the three residential areas where outdoor concentrations were higher than those indoors (Table 4). However, the difference was not statistically significant (Old Sicelo p = 0.23; New Sicelo p = 0.35; Noldick p = 0.52). A statistically insignificant difference was also found when only the indoor concentrations across the three residential areas were compared using one-way ANOVA (p = 0.95) and when comparing the outdoor PM2.5-bound-metal(loid) concentrations (p = 0.81). The R-square values presented in Table 5 showed that 96% of the variance in indoor PM2.5-bound metal(loid) concentrations in Old Sicelo can be accounted for by outdoor concentrations, while 92% of the variance in indoor PM2.5-bound metal(loid)s concentrations in New Sicelo can be accounted for by the outdoor concentrations. In Noldick, 81% of the variance in indoor PM2.5-bound metal(loid)s concentrations can be accounted for by outdoor concentrations.
Health risk analysis
The risk of developing non-carcinogenic health effects through inhalation when indoors and outdoors for the four age groups is shown in Figs. 2 and 3. Notably, the HQ of Mn was >1 both indoors and outdoors for the four age groups across the three residential areas, indicating a risk of developing non-carcinogenic health effects. The HQs of Cr (VI) displayed a similar trend for all age groups; it was >1 in all residential areas except for outdoor environments at New Sicelo. For Co, the HQ was >1 only outdoors at Old Sicelo for all age groups, except for the 21–35 age group. The HQs of Mn and Cr (VI) were higher both indoors and outdoors at Noldick for all age groups compared to the other residential areas. The highest HQs for Mn were recorded indoors and outdoors at Noldick for the 36–65+ age group, whereas the lowest was recorded at Old Sicelo for the 21–35 age group. For example, the Mn HQ at outdoor environments in Noldick for the 36–65+ age group was 16-fold greater than that of the 21–35 age group at Old Sicelo.
Figure 4 shows the overall risk of developing non-carcinogenic health effects because of exposure to PM2.5-bound metal(loid)s through inhalation. The overall exposure was obtained using Eq. (2). As shown in Fig. 4, only the overall HQ of Mn >1 for the four age groups across the three residential areas indicated the risk of developing non-carcinogenic health effects. The overall HQs of Mn and Cr (VI) were higher at Noldick for all age groups implying that the population in Noldick is at the highest risk of developing adverse health effects. Similar to the indoor and outdoor HI, the overall HI was also >1 for the four age groups across the three residential areas, indicating a risk of developing non-carcinogenic health effects.
The risk of developing cancer due to exposure to the metal(loid)s through the inhalation route for age groups of 1–5 and 6–20 years is shown in Table 6. For the 1–5 years age group, the cancer risk for Cr (VI) was above the acceptable limit both indoors and outdoors across the three residential areas. The cancer risk for Co was above the minimum acceptable limit of 1 × 10−6 both indoors and outdoors only at Old Sicelo and indoors in New Sicelo and Noldick. For Cd, the cancer risk was above the acceptable limit only in the indoor environments across the three residential areas. For the 6–20 age group, the cancer risk for Cr (VI) was above the acceptable limit of 1 × 10−4 both indoors and outdoors at New Sicelo and only in indoor environments at Old Sicelo and Noldick. For Co, the cancer risk was above the acceptable limit of 1 × 10−6 in indoor environments in Old Sicelo and above the acceptable limit of 1 × 10−5 in outdoor environments. Furthermore, the cancer risk for Co was above the acceptable limit of 1 × 10−6 in outdoor environments at New Sicelo and Noldick. The cancer risk for Cd for the 6–20 years age group was above the acceptable limit of 1 × 10−6 both indoors and outdoors across the three residential areas.
Table 7 shows the risk of developing cancer in the 21–35 and 36–65+ years age groups. As shown in Table 6, the cancer risk for Cr (VI) was found both indoors and outdoors only at New Sicelo for the 21–35 years age group. The cancer risk for Co was found both indoors (>1 × 10−6) and outdoors (>1 × 10−5) at Old Sicelo, while at New Sicelo and Noldick, the cancer risk (>1 × 10−6) was found indoor environments. For Cd, the cancer risk was above the acceptable limit of 1 × 10−6, both indoors and outdoors, across the three residential areas. For the 36–65+ age group, the cancer risk for Cr (VI) was found (>1 × 10−5) only in outdoor environments at New Sicelo. Regarding the cancer risk for Co, there was a cancer risk both indoors and outdoors across the three residential areas. However, the cancer risk was above the minimum acceptable limit of 1 × 10−5 in all indoor environments, above 1 × 10−6 in outdoor environments at New Sicelo and Noldick, and above 1 × 10−5 outdoor environments at Old Sicelo. The cancer risk for Cd was above the minimum acceptable limit of 1 × 10−5 indoors and above 1 × 10−6 in outdoor environments across the three residential areas.
Table 8 shows the overall risk of carcinogenic health effects across the three residential areas for the four age groups. A cancer risk was found for Cr (VI) for all age groups across the residential areas, except for the 6–20 age group at Old Sicelo. For the 1–5 years age group, the cancer risk for Cr (VI) was above the minimum acceptable limit of 1 × 10−5, whereas the cancer risk for the 6–20 years age group was found at New Sicelo (>1 × 10−5) and Noldick (>1 × 10−4). The cancer risk for the 21–35 and 36–65+ age groups was similar across the three residential areas. At Old Sicelo and Noldick, the cancer risk was above the minimum acceptable limit of 1 × 10−4, whereas at New Sicelo it was above 1 × 10−5. For Co, cancer risk was found (>1 × 10−6) for the 6–20 and 21–35 years age groups at Old Sicelo.
Discussion
Indoor and outdoor PM2.5 elemental composition
The PM2.5 elemental composition results presented in Table 4 showed that outdoor Mn concentrations were higher at Noldick, followed by New Sicelo, which are both within 1.5 km from the ferromanganese smelter. The lowest concentrations were found at Old Sicelo which was approximately 3.5 km away. These findings indicate that the population living nearest to ferromanganese smelters is at the highest risk of exposure and developing adverse health effects. The higher concentrations of Mn, particularly indoors, are a public health concern because people spend 80–90% of their time in indoor microenvironments where exposure is likely to occur and given the health effects of exposure to Mn through inhalation (Morawska et al. 2013).
The indoor and outdoor PM2.5-bound metal(loid)s found in this study have been reported in other studies conducted in residential areas near ferromanganese smelters and have been confirmed to be commonly emitted from ferromanganese smelters. Hernández-Pellón and Fernández-Olmo (2019b) found elevated maximum ambient daily concentrations of Mn (2061.6 ng/m3) in an area near a manganese alloy plant. Racette et al. (2021a) reported that a 2-year average PM2.5-bound Mn concentration at Noldick was 203 ng/m3, which was almost 2-fold higher than those of Old Sicelo and New Sicelo. Hernández-Pellón and Fernández-Olmo (2019a) and Hernández-Pellón et al. (2017) conducted studies near a ferromanganese alloy plant in Spain and reported an abundance of PM10-bound metal(loid)s in ambient samples. Specifically, they found an abundance of Mn- and Fe-enriched PM10.
The fact that the airborne PM2.5-bound Mn concentrations in this study are comparable to those of previous studies conducted near ferromanganese smelters indicates that the ferromanganese smelter in Meyerton is a major contributing source of PM2.5-bound metal(loid)s. However, the outdoor PM2.5-bound metal(loid) concentrations reported in this study were higher than those reported by Hernández-Pellón and Fernández-Olmo (2019a) in a study conducted in three residential areas downwind and within 10 km of a ferromanganese smelter in Cantabria, Spain. The present results are also higher than those of Racette et al. (2021a), conducted in the same area. The differences in outdoor concentrations might be attributed to the sampling methodology, sampling devices, and environmental conditions. Since most studies conducted in residential areas near ferromanganese smelters focused on outdoor concentrations, it is difficult to compare the indoor findings. Nonetheless, in a study conducted in three homes in Nanjing, China, Wang et al. (2018) found that the concentration of PM2.5-bound metal(loid)s outdoors was significantly greater than that indoors.
Relationship between indoor and outdoor PM2.5-bound metal(loid)s
Although there was variation between the concentrations of indoor and outdoor PM2.5-bound metal(loid)s, a positive linear relationship was found between the indoor and outdoor concentrations in the three residential areas. As presented in Table 5, the correlation coefficient (multiple R) in the three residential areas ranged between 90 and 98, indicating a strong positive linear relationship between the concentrations of indoor and outdoor PM2.5-bound metal(loid)s. Furthermore, the linear relationship between the indoor and outdoor concentrations was statistically significant (p < 0.05). The results are not surprising given that 35–70% of indoor PM is from the outdoor environment (Lv et al. 2017) which enters indoor environments through infiltration, ventilation, or filtration mechanisms, openings and improperly sealed windows (Chen and Zhao 2011) and through foot tracking (Lee et al. 2002).
Health risk analysis
The HRA findings indicated that the Noldick population, approximately 1.4 km from the ferromanganese smelter, is at the highest risk of developing non-carcinogenic health effects compared to the population in Old and New Sicelo. Moreover, the 36–65+ age group is at a higher risk of developing non-carcinogenic health effects owing to exposure to Mn through the inhalation route. The HI values of all metal(loid)s were >1 for all age groups both indoors and outdoors across the three residential areas, indicating a risk of developing non-carcinogenic health effects due to exposure to multiple metal(loid)s via inhalation. From Fig. 4, it can be noted that when using the overall exposure concentrations of the PM2.5-bound metal(loid)s obtained using Eq. (2), the HQ and HI were lower than when using the indoor and outdoor concentrations.
Hernández-Pellón et al. (2018) reported a HQ > 1 for Mn, particularly when using a worst-case scenario in a study conducted near an industrial area with a ferroalloy plant in Cantabria, northern Spain. The HQs of Mn, Cr (VI), and Cd which were >1 are a significant public health concern considering the irreversible negative health effects associated with exposure to these metal(loid)s. Compelling evidence from epidemiological studies has revealed an association between Mn exposure through inhalation and behavioural disorders (Rodrigues et al. 2018a), reduced performance in learning activities and verbal memory (Carvalho et al. 2018), and impaired motor function (Bowler et al. 2016), which are more pronounced in children than in adults (Rodrigues et al. 2018b). Cadmium is excreted at a slow rate in the human system; hence, it has a half-life of up to 20 years (Keil et al. 2011). Furthermore, Cd is an endocrine disruptor and long-term exposure to Cd can cause cancer (Jiang et al. 2009). Exposure to Cd at low concentrations can cause pathways that trigger cell proliferation and hinder cell growth at higher concentrations (Park et al. 2021). Exposure to Cr is associated with lung cancer, nasal irritation, and perforation. Furthermore, animal studies have shown that exposure to Cr (VI) is associated with tissue damage and inflammation (Proctor et al. 2014).
Similar to the HQ and HI values, the cancer risk values were lower when using the overall exposure concentrations of PM2.5-bound metal(loid)s than when using the indoor and outdoor concentrations. It should be noted that the acceptable cancer limits are for regulatory purposes; therefore, a no risk of cancer does not necessarily mean the Meyerton population are safe from developing carcinogenic health effects. Furthermore, the development of adverse health effects depends on the individual’s characteristics and social status. Risk estimation has limitations that might underestimate the associated health risks.
Strengths and limitations
This is the first HRA study conducted near a ferromanganese smelter in RSA. Furthermore, the study used indoor and outdoor PM2.5-bound metal(loid)s to determine the risk of developing carcinogenic and non-carcinogenic health effects instead of focusing on the concentration from one microenvironment. Furthermore, the study did not use data obtained from fixed monitoring stations and instead used data collected at the level of the receptor both indoors and outdoors. Additionally, the overall exposure concentration was calculated to determine the health risks of exposure to both indoor and outdoor PM2.5-bound metal(loid)s.
This study has some limitations. The sampling time was limited to 3 months; therefore, the results might not be a true representation of the exposure and potential for developing adverse health effects for the Meyerton population. Furthermore, data were only collected in spring; however, it is unlikely that the indoor and outdoor PM2.5-bound metal(loid) concentrations could remain the same throughout the seasons due to environmental conditions. Therefore, the results must be interpreted with caution since the sampling period was short and over one season. Although the PM2.5 concentrations used were collected at the level of the receptor rather than using fixed monitoring stations, data collected from the breathing zone would have been a representation. Haynes et al. (2012) found that personal PM2.5 concentrations were consistently greater than those obtained from stationery monitors, indicating that data not obtained from the breathing zone can underestimate exposure.
The exposure concentration was used to calculate the average daily dose instead of the deposited dose. Adverse health effects are due to the retained dose and not the exposure concentration; therefore, it is better to use this dose than the exposure concentration. Furthermore, the health risk assessment was based on the total concentration of metal(loid)s and not the bioaccessible concentration, which is the fraction of metal(loid)s that can be solubilised by a human synthetic fluid (Markiv et al. 2022). According to Hernández-Pellón et al. (2018), using the total metal(loid) concentration may overestimate health risks. The HRA did not consider the sex of the different age groups. Dong et al. (2011) found that the health implications of exposure to air pollution differ by sex; specifically, females are more susceptible than males and the effects are more severe in females than males. Furthermore, this study used general life expectancy to calculate the non-carcinogenic health effects; however, the life expectancy of males and females in RSA differs significantly. For example, the life expectancy of females in RSA is 1.1-fold greater than that of males.
Uncertainties
The health risk assessment was based on assumptions and values that might not precisely represent the Meyerton population. The HQ values for K, Fe, Mg, and Na were not calculated because of the unavailability of RfCs. Nonetheless, these metal(loid)s are trace elements which are essential for body functions but can cause excessive levels of health problems (Taner et al. 2013). The metal(loid)s were not speciated and studies have shown that the health implications of exposure to metal(loid)s are influenced by their physical properties. For example, the toxicity of Cr is influenced by its valence state (Cr (III) or Cr (VI)) (Karim et al. 2015). Furthermore, the toxicity of Cr (VI) is greater than that of Cr (III); hence, it has been categorised as a class 2B carcinogen (Wang et al. 2011). The indoor and outdoor concentrations of Cd and K were adjusted since they were below the LoQ. The values of K and Cd were the same both indoors and outdoors in all the residential areas. Subsequently, the HQ values for Cd were the same indoors and outdoors in all residential areas. The exposure scenarios were based on the average time spent indoors obtained from studies conducted in European countries owing to the lack of available time activity pattern data in RSA. It is unlikely that South Africans spend 80–90% of their time indoors; therefore, time-activity patterns might not represent the population in Meyerton.
Risk management and recommendations
Risk management is the last stage of a risk assessment used to recommend and implement mitigation measures to prevent exposure and the potential for developing adverse health effects where non-carcinogenic and carcinogenic health risks are found. The health risk findings presented in this study are concerning and therefore require urgent intervention from relevant authorities. Previous epidemiological studies conducted in this area have found an association between Mn exposure and adverse health effects, some of which are irreversible. Racette et al. (2021b) investigated the severity of parkinsonism among residents from the age of 40 and above in Meyerton. The authors concluded that long-term exposure to airborne Mn concentrations, even at significantly lower concentrations, may be linked to clinical parkinsonism. Another study (Racette et al. 2021a) investigating depression and anxiety among the Meyerton population aged 40 years and above found a link between mood and long-term exposure to Mn at concentrations lower than those reported in similar previous studies. The authors concluded that exposure to airborne Mn in residential areas might have health implications beyond impaired motor system pathways. Furthermore, there is no exposure threshold for carcinogens, below which there is no risk of developing carcinogenic health effects in a lifetime (De Donno et al. 2018).
Considering the present study’s findings and those of previous epidemiological studies conducted in the Meyerton area, it is important to implement effective interventions to protect public health. The starting point of implementing mitigation measures is to ensure that the ferromanganese smelter complies with the South African national air quality standards and that control measures are in place to reduce emissions. Efforts must be made to provide decent housing because most residents, especially in New and Old Sicelo, stay in shacks that can enhance the infiltration and penetration of outdoor PM into indoor environments. Furthermore, trees must be planted in residential areas near and downwind of ferromanganese smelters. The trees help trap and absorb some of the pollutants via occult deposition, thus enhancing the air quality in the areas. Residential areas without paving or tar roads must be provided to prevent the resuspension of settled PM by vehicles, humans, and wind. In future, residential areas should not be developed in industries such as ferromanganese smelters; therefore, there is a need for stringent town planning. There is a need for awareness and education about the risks associated with exposure to PM-bound metal(loid)s, and measures that can be taken to prevent exposure in such residential areas. The use of air purifiers to remove pollutants from indoor environments must be promoted. For example, high-efficiency particulate air (HEPA) filters are effective at removing fine particles (Dubey et al. 2021).
Conclusion
For the first time in RSA, a HRA assessment using both indoor and outdoor PM2.5-bound metal(loid) concentrations was conducted in residential areas downwind of a ferromanganese smelter. The concentrations of PM2.5-bound metal(loid)s in the three residential areas were higher outdoors than in the indoor environment, and the dominant metal(loid)s were in the order of K > Fe > Si > Mn > Mg. Populations living near and downwind of ferromanganese smelters are at risk of developing carcinogenic and non-carcinogenic health effects. The findings indicate that the Noldick population, which is approximately 1.4 km from the ferromanganese smelter, is at the highest risk of developing non-carcinogenic health effects compared with the population in Old and New Sicelo. The HQ values for Mn and Cr (VI) were >1, indicating the risk of developing non-carcinogenic health effects. The 36–65+ age group in Noldick was at the highest risk of developing non-carcinogenic health effects owing to exposure to Mn. The cancer risk values of Cr (VI), Co, and Cd exceeded the recommended upper limit of 1 × 10−4 and the lower limit of 1 × 10−6. However, using the overall concentrations yielded a lower cancer risk and HQ and HI values. This study showed that it is important to consider indoor microenvironments because they are where people spend most of their time and are likely to be exposed to a mixture of indoor and outdoor PM2.5-bound metal(loid)s. Future studies must use data obtained from the breathing zone, and the bioaccessible or bioavailable concentration must be used to determine health risks instead of the total concentration of metal(loid)s. This is important because metal(loid)s that can be solubilised in biological fluids can enter the blood circulation and cause damaging health effects. Furthermore, epidemiological studies using biomarkers of exposure, such as hair, saliva, and blood samples, and biomarkers of effects, such as magnetic resonance imaging scans in the population near the ferromanganese smelter, are required to corroborate the study findings. Future studies in this area must conduct speciation since the toxicity of metal(loid)s is influenced by their chemical speciation.
Data availability
The raw data related to this research may be provided on reasonable request from the corresponding author.
References
Abdel-Salam MMM (2022) Indoor exposure of elderly to air pollutants in residential buildings in Alexandria, Egypt. Build Environ 219:109221. https://doi.org/10.1016/j.buildenv.2022.109221
Ali MU, Lin S, Yousaf B et al (2022) Pollution characteristics, mechanism of toxicity and health effects of the ultrafine particles in the indoor environment: current status and future perspectives. Crit Rev Environ Sci Technol 52:436–473. https://doi.org/10.1080/10643389.2020.1831359
Bollati V, Marinelli B, Apostoli P et al (2010) Exposure to metal-rich particulate matter modifies the expression of candidate microRNAs in peripheral blood leukocytes. Environ Health Perspect 118:763–768. https://doi.org/10.1289/ehp.0901300
Bornhorst J, Wehe CA, Hüwel S et al (2012) Impact of manganese on and transfer across blood-brain and blood-cerebrospinal fluid barrier in vitro. J Biol Chem 287:17140–17151. https://doi.org/10.1074/jbc.M112.344093
Boudissa SM, Lambert J, Müller C et al (2006) Manganese concentrations in the soil and air in the vicinity of a closed manganese alloy production plant. Sci Total Environ 361:67–72. https://doi.org/10.1016/j.scitotenv.2005.05.001
Bowler RM, Beseler CL, Gocheva VV et al (2016) Environmental exposure to manganese in air: associations with tremor and motor function. Sci Total Environ 541:646–654. https://doi.org/10.1016/j.scitotenv.2015.09.084
Brokamp C, Rao MB, Fan Z, Ryan PH (2015) Does the elemental composition of indoor and outdoor PM2.5 accurately represent the elemental composition of personal PM2.5? Atmos Environ 101:226–234. https://doi.org/10.1016/j.atmosenv.2014.11.022
Calderón J, Ortiz-Pérez D, Yáñez L, Dıiaz-Barriga F (2003) Human exposure to metals. Pathways of exposure, biomarkers of effect, and host factors. Ecotoxicol Environ Saf 56:93–103. https://doi.org/10.1016/S0147-6513(03)00053-8
Carvalho CF, Oulhote Y, Martorelli M et al (2018) Environmental manganese exposure and associations with memory, executive functions, and hyperactivity in Brazilian children. Neurotoxicology 69:253–259. https://doi.org/10.1016/j.neuro.2018.02.002
Castro A, Götschi T, Achermann B et al (2020) Comparing the lung cancer burden of ambient particulate matter using scenarios of air quality standards versus acceptable risk levels. Int J Public Health 65:139–148. https://doi.org/10.1007/s00038-019-01324-y
Chen C, Zhao B (2011) Review of relationship between indoor and outdoor particles: I/O ratio, infiltration factor and penetration factor. Atmos Environ 45:275–288. https://doi.org/10.1007/s11356-016-6826-7
Creamer M (2013) New R1bn manganese furnace signals beneficiation support – BHP Billiton. Eng. News Min. Wkly. https://m.miningweekly.com/article/new-r1bn-manganese-furnace-signals-support-for-beneficiation-bhp-billiton-2013-03-06. Accessed 2 Jul 2020
Davourie J, Westfall L, Ali M, Mcgough D (2017) Evaluation of particulate matter emissions from manganese alloy production using life-cycle assessment. Neurotoxicology 58:180–186. https://doi.org/10.1016/j.neuro.2016.09.015
De Donno A, De Giorgi M, Bagordo F et al (2018) Health risk associated with exposure to PM10 and benzene in three Italian towns. Int J Environ Res Public Health 15:1672. https://doi.org/10.3390/ijerph15081672
Delgado-Saborit JM (2019) Indoor air as a contributor to air pollution exposure. In: Issues in environmental science and technology. The Royal Society of Chemistry, pp 158–195. https://doi.org/10.1039/9781788016179-00158
Demirel G, Özden Ö, Döğeroğlu T, Gaga EO (2014) Personal exposure of primary school children to BTEX, NO2 and ozone in Eskişehir, Turkey: relationship with indoor/outdoor concentrations and risk assessment. Sci Total Environ 473–474:537–548. https://doi.org/10.1016/j.scitotenv.2013.12.034
Dong G-H, Chen T, Liu M-M et al (2011) Gender differences and effect of air pollution on asthma in children with and without allergic predisposition: northeast Chinese children health study. PLoS One 6:e22470. https://doi.org/10.1371/journal.pone.0022470
Dubey S, Rohra H, Taneja A (2021) Assessing effectiveness of air purifiers (HEPA) for controlling indoor particulate pollution. Heliyon 7:e07976. https://doi.org/10.1016/j.heliyon.2021.e07976
Expósito A, Markiv B, Ruiz-Azcona L et al (2021) Personal inhalation exposure to manganese and other trace metals in an environmentally exposed population: bioaccessibility in size-segregated particulate matter samples. Atmos Pollut Res 12:101123. https://doi.org/10.1016/j.apr.2021.101123
Fernández-Camacho R, Rodríguez S, de la Rosa J et al (2012) Ultrafine particle and fine trace metal (As, Cd, Cu, Pb and Zn) pollution episodes induced by industrial emissions in Huelva, SW Spain. Atmos Environ 61:507–517. https://doi.org/10.1016/j.atmosenv.2012.08.003
Fiordelisi A, Piscitelli P, Trimarco B et al (2017) The mechanisms of air pollution and particulate matter in cardiovascular diseases. Heart Fail Rev 22:337–347. https://doi.org/10.1007/s10741-017-9606-7
Geiser M, Rothen-Rutishauser B, Kapp N et al (2005) Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ Health Perspect 113:1555–1560. https://doi.org/10.1289/ehp.8006
Goix S, Lévêque T, Xiong T-T et al (2014) Environmental and health impacts of fine and ultrafine metallic particles: assessment of threat scores. Environ Res 133:185–194. https://doi.org/10.1016/j.envres.2014.05.015
Greig AJ (2000) Monitoring airborne particles as mass and number concentrations: implications for air quality management and health. Int J Environ Stud 57:641–662. https://doi.org/10.1080/00207230008711302
Hammer SE, Ervik T, Ellingsen DG et al (2021) Particle characterisation and bioaccessibility of manganese in particulate matter in silico- and ferromanganese smelters. Environ Sci Process Impacts 23:1488–1499. https://doi.org/10.1039/D1EM00243K
Haynes EN, Ryan P, Chen A et al (2012) Assessment of personal exposure to manganese in children living near a ferromanganese refinery. Sci Total Environ 427–428:19–25. https://doi.org/10.1016/j.scitotenv.2012.03.037
Hernández-Pellón A, Fernández-Olmo I (2019a) Airborne concentration and deposition of trace metals and metalloids in an urban area downwind of a manganese alloy plant. Atmos Pollut Res 10:712–721. https://doi.org/10.1016/j.apr.2018.11.009
Hernández-Pellón A, Fernández-Olmo I (2019b) Using multi-site data to apportion PM-bound metal(loid)s: impact of a manganese alloy plant in an urban area. Sci Total Environ 651:1476–1488. https://doi.org/10.1016/j.scitotenv.2018.09.261
Hernández-Pellón A, Fernández-Olmo I (2016) Monitoring the levels of particle matter-bound manganese: an intensive campaign in an urban/industrial area. In: In: 2nd international conference on atmospheric dust. Digilabs, pp 50–55. https://doi.org/10.14644/dust.2016.008
Hernández-Pellón A, Fernández-Olmo I, Ledoux F et al (2017) Characterization of manganese-bearing particles in the vicinities of a manganese alloy plant. Chemosphere 175:411–424. https://doi.org/10.1016/j.chemosphere.2017.02.056
Hernández-Pellón A, Nischkauer W, Limbeck A, Fernández-Olmo I (2018) Metal(loid) bioaccessibility and inhalation risk assessment: a comparison between an urban and an industrial area. Environ Res 165:140–149. https://doi.org/10.1016/j.envres.2018.04.014
Hinds WC (1999) Aerosol technology: properties, behaviour, and measurement of airborne particles, 2nd edn. Wiley-interscience, New York, NY
Hornung RW, Reed LD (1990) Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 5:46–51. https://doi.org/10.1080/1047322X.1990.10389587
Jiang G, Duan W, Xu L et al (2009) Biphasic effect of cadmium on cell proliferation in human embryo lung fibroblast cells and its molecular mechanism. Toxicol Vitr 23:973–978. https://doi.org/10.1016/j.tiv.2009.06.029
Karim Z, Qureshi BA, Mumtaz M (2015) Geochemical baseline determination and pollution assessment of heavy metals in urban soils of Karachi, Pakistan. Ecol Indic 48:358–364. https://doi.org/10.1016/j.ecolind.2014.08.032
Keil DE, Berger-Ritchie J, McMillin GA (2011) Testing for toxic elements: a focus on arsenic, cadmium, lead, and mercury. Lab Med 42:735–742. https://doi.org/10.1309/LMYKGU05BEPE7IAW
Kelly FJ, Fussell JC (2012) Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos Environ 60:504–526. https://doi.org/10.1016/j.atmosenv.2012.06.039
Kim CS, Kang TC (1997) Comparative measurement of lung deposition of inhaled fine particles in normal subjects and patients with obstructive airway disease. Am J Respir Crit Care Med 155:899–905. https://doi.org/10.1164/ajrccm.155.3.9117024
Kim K-H, Kabir E, Kabir S (2015) A review on the human health impact of airborne particulate matter. Environ Int 74:136–143. https://doi.org/10.1016/j.envint.2014.10.005
Lee SC, Guo H, Li WM, Chan LY (2002) Inter-comparison of air pollutant concentrations in different indoor environments in Hong Kong. Atmos Environ 36:1929–1940. https://doi.org/10.1016/S1352-2310(02)00176-0
Legay C, Rodriguez MJ, Sadiq R et al (2011) Spatial variations of human health risk associated with exposure to chlorination by-products occurring in drinking water. J Environ Manage 92:892–901. https://doi.org/10.1016/j.jenvman.2010.10.056
Löndahl J, Massling A, Pagels J et al (2007) Size-resolved respiratory-tract deposition of fine and ultrafine hydrophobic and hygroscopic aerosol particles during rest and exercise. Inhal Toxicol 19:109–116. https://doi.org/10.1080/08958370601051677
Lovaković BT, Jagić K, Dvoršćak M, Klinčić D (2022) Trace elements in indoor dust—children’s health risk considering overall daily exposure. Indoor Air 32(9):e13104. https://doi.org/10.1111/ina.13104
Lv Y, Wang H, Wei S et al (2017) The correlation between indoor and outdoor particulate matter of different building types in Daqing, China. Procedia Eng 205:360–367. https://doi.org/10.1016/j.proeng.2017.10.002
Mannucci PM, Franchini M (2017) Health effects of ambient air pollution in developing countries. Int J Environ Res Public Health 14:1–9. https://doi.org/10.3390/ijerph14091048
Mansha M, Ghauri B, Rahman S, Amman A (2012) Characterization and source apportionment of ambient air particulate matter (PM2.5) in Karachi. Sci Total Environ 425:176–183. https://doi.org/10.1016/j.scitotenv.2011.10.056
Markiv B, Ruiz-Azcona L, Expósito A et al (2022) Short- and long-term exposure to trace metal(loid)s from the production of ferromanganese alloys by personal sampling and biomarkers. Environ Geochem Health 44:4595–4618. https://doi.org/10.1007/s10653-022-01218-8
Matooane M, Diab R (2003) Health risk assessment for sulfur dioxide pollution in south Durban, South Africa. Arch Environ Heal An Int J 58:763–770. https://doi.org/10.3200/AEOH.58.12.763-770
Mbazima SJ (2022) Health risk assessment of particulate matter 2.5 in an academic metallurgy workshop. Indoor Air 32(9):e13111. https://doi.org/10.1111/ina.13111
Mbazima SJ, Masekameni MD, Nelson G (2021) Physicochemical properties of indoor and outdoor particulate matter 2.5 in selected residential areas near a ferromanganese smelter. Int J Environ Res Public Health 18:1-18. https://doi.org/10.3390/ijerph18178900
Monn C (2002) Exposure assessment of air pollutants: a review on spatial heterogeneity and indoor/outdoor/personal exposure to suspended particulate matter, nitrogen dioxide and ozone. Atmos Environ 35:1–32. https://doi.org/10.1016/S1474-8177(02)80007-9
Morawska L, Afshari A, Bae GN et al (2013) Indoor aerosols: from personal exposure to risk assessment. Indoor Air 23:462–487. https://doi.org/10.1111/ina.12044
Morawska L, Ayoko GA, Bae GN et al (2017) Airborne particles in indoor environment of homes, schools, offices and aged care facilities: the main routes of exposure. Environ Int 108:75–83. https://doi.org/10.1016/j.envint.2017.07.025
Neris JB, Olivares DMMM, Velasco FG et al (2019) HHRISK: a code for assessment of human health risk due to environmental chemical pollution. Ecotoxicol Environ Saf 170:538–547. https://doi.org/10.1016/j.ecoenv.2018.12.017
Park E, Kim S, Song S-H et al (2021) Environmental exposure to cadmium and risk of thyroid cancer from national industrial complex areas: a population-based cohort study. Chemosphere 268:128819. https://doi.org/10.1016/j.chemosphere.2020.128819
Pearson GF, Greenway GM (2005) Recent developments in manganese speciation. TrAC Trends Anal Chem 24:803–809. https://doi.org/10.1016/j.trac.2005.02.008
Proctor DM, Suh M, Campleman SL, Thompson CM (2014) Assessment of the mode of action for hexavalent chromium-induced lung cancer following inhalation exposures. Toxicology 325:160–179. https://doi.org/10.1016/j.tox.2014.08.009
Racette BA, Nelson G, Dlamini WW et al (2021a) Depression and anxiety in a manganese-exposed community. Neurotoxicology 85:222–233. https://doi.org/10.1016/j.neuro.2021.05.017
Racette BA, Nelson G, Dlamini WW et al (2021b) Severity of parkinsonism associated with environmental manganese exposure. Environ Heal 20:27. https://doi.org/10.1186/s12940-021-00712-3
Rodrigues JLG, Araújo CFS, dos Santos NR et al (2018a) Airborne manganese exposure and neurobehavior in school-aged children living near a ferro-manganese alloy plant. Environ Res 167:66–77. https://doi.org/10.1016/j.envres.2018.07.007
Rodrigues JLG, Bandeira MJ, Araújo CFSF et al (2018b) Manganese and lead levels in settled dust in elementary schools are correlated with biomarkers of exposure in school-aged children. Environ Pollut 236:1004–1013. https://doi.org/10.1016/j.envpol.2017.10.132
Roy R (2016) The cost of air pollution in Africa. www.oecd.org/dev/wp. Accessed 14 Mar 2022
Schwartz J, Laden F, Zanobetti A (2002) The concentration-response relation between PM 2.5 and daily deaths. Environ Heal Perspect 110(10):1025–1029
Sly PD, Carpenter DO, Van den Berg M et al (2016) Health consequences of environmental exposures: causal thinking in global environmental epidemiology. Ann Glob Heal 82:3. https://doi.org/10.1016/j.aogh.2016.01.004
Solís-Vivanco R, Rodríguez-Agudelo Y, Riojas-Rodríguez H et al (2009) Cognitive impairment in an adult Mexican population non-occupationally exposed to manganese. Environ Toxicol Pharmacol 28:172–178. https://doi.org/10.1016/j.etap.2009.04.001
Statistics South Africa (2011) Statistics South Africa. http://www.statssa.gov.za/?page_id=4286&id=11181. Accessed 20 Oct 2019
Statistics South Africa (2021) Mid-year population estimates 2021. Tshwane. https://www.statssa.gov.za/?page_id=1854&PPN=P0302&SCH=72983. Accessed Nov 2022
Steyn L (2018) South32 reviews future of smelter amid higher power prices. Business Day. https://www.businesslive.co.za/bd/companies/mining/2019-07-18-south32-reviews-future-of-smelter-amid-higher-power-prices/. Accessed 02 Jul 2020
Straif K, Benbrahim-Tallaa L, Baan R et al (2009) A review of human carcinogens—part C: metals, arsenic, dusts, and fibres. Lancet Oncol 10:453–454. https://doi.org/10.1016/S1470-2045(09)70134-2
Taner S, Pekey B, Pekey H (2013) Fine particulate matter in the indoor air of barbeque restaurants: elemental compositions, sources and health risks. Sci Total Environ 454–455:79–87. https://doi.org/10.1016/j.scitotenv.2013.03.018
Tsuda A, Henry FS, Butler JP (2013) Particle transport and deposition: basic physics of particle kinetics. Compr Physiol 3:1437–1471. https://doi.org/10.1002/cphy.c100085
United States Environmental Protection Agency (2009) Risk assessment guidance for superfund volume I: human health evaluation manual (part F, supplemental guidance for inhalation risk assessment). United States Environmental Protection Agency, Washington DC. https://www.epa.gov/risk/risk-assessment-guidance-superfund-rags-part-f. Accessed 1 Aug 2020
United States Environmental Protection Agency (2000) Risk characterization handbook. United States Environmental Protection Agency, Washington, DC. https://www.epa.gov/sites/default/files/2015-10/documents/osp_risk_characterization_handbook_2000.pdf. Accessed 17 Feb 2018
Wang F, Zhou Y, Meng D et al (2018) Heavy metal characteristics and health risk assessment of PM2.5 in three residential homes during winter in Nanjing, China. Build Environ 143:339–348. https://doi.org/10.1016/j.buildenv.2018.07.011
Wang Z-X, Chen J-Q, Chai L-Y et al (2011) Environmental impact and site-specific human health risks of chromium in the vicinity of a ferro-alloy manufactory, China. J Hazard Mater 190:980–985. https://doi.org/10.1016/j.jhazmat.2011.04.039
World Health Organization (2016) Ambient air pollution: a global assessment of exposure and burden of disease. World Health Organization, Geneva. https://www.who.int/publications/i/item/9789241511353. Accessed 3 Mar 2019
Acknowledgements
The author wishes to thank all the Meyerton residents that participated in this; the study would have not been a success without the cooperation and permission to collect from their houses. Many thanks to Tony da Silva for helping navigate Meyerton and assisting with the data collection. The author also acknowledges the occupational hygiene division at the National Institute for Occupational Health for their support with sampling equipment, and Petrus Philipus Pieterse from the University of Johannesburg Spectrum Laboratory for assisting with the ICP-MS analysis. Appreciation to Yonda “YoYo” Nokhwethu for proofreading the manuscript. Lastly, the author extends appreciation to the anonymous reviewers for the constructive comments they provided to improve the quality of the earlier version of this manuscript.
Funding
Open access funding provided by University of the Witwatersrand.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Human Research Ethics Committee at the University of the Witwatersrand, Johannesburg (clearance certificate no: M150466, 15/06/2015).
Consent to participate
Informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The author declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mbazima, S.J. Health risk assessment of indoor and outdoor PM2.5-bound metal(loid)s in three residential areas downwind of an active ferromanganese smelter. Air Qual Atmos Health 16, 2309–2323 (2023). https://doi.org/10.1007/s11869-023-01409-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-023-01409-x