Opinion statement
Nasopharyngeal carcinoma (NPC) is a rare malignancy, endemic in China, that is commonly diagnosed in locally advanced scenarios. Its pathogenesis is strongly associated with Epstein-Barr virus (EBV), an infection for which measuring EBV plasma DNA levels has helped as a prognostic factor guiding treatment options, including a stronger treatment in those with high titers. Additionally, tobacco and alcohol are often implicated in EBV-negative patients. The local disease is treated with radiotherapy alone, preferentially intensity modulated radiotherapy. For locally advanced disease, the backbone treatment is concurrent chemoradiotherapy with the ongoing research dilemma being adding adjuvant chemotherapy or induction chemotherapy. The ongoing research is focused not only on identifying patients that will benefit from adjuvant or induction chemotherapy, but also on identifying the best chemotherapeutic regimen, regimen alternatives to diminish toxicity, the role that immune checkpoint inhibitors play, and the use of molecularly guided treatment targeting patients with NPC whether driven by EBV or tobacco and alcohol. Knowing the precise oncogenesis of NPC not only offers a better understanding of the role that EBV plays in this tumor but also helps create targeted therapies that could potentially block important pathways such as the NF-κB pathway. Much is yet to be done, but the prognosis and management of NPC patients have changed drastically, offering precise treatment methods and excellent control of the disease, even in locally advanced scenarios.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasopharyngeal carcinoma (NPC) is a rare malignancy with age-standardized rates worldwide below 1 per 100,000 person-years [1]. In 2021, a total of 133,000 new cases were diagnosed, and 80,000 deaths occurred due to nasopharyngeal carcinoma [2]. However, NPC is common in Southern China, especially in the Cantonese population, with an increased incidence rate of up to 50-fold between Northern and Southern China [3]. NPC has a 2–3 times higher incidence in males compared to females, with a higher incidence peak between 50 and 59 years of age [4].
The World Health Organization (WHO) classifies NPC into three subtypes [5, 6]: (1) squamous keratinizing cell carcinoma associated with tobacco exposure; (2) non-keratinizing squamous cell carcinoma potentially viral (EBV and human papillomavirus [HPV]) or tobacco-related; (3) undifferentiated or poorly differentiated carcinoma (lymphoepithelial variants) that are EBV related. NPC’s most common histology in high-incidence areas is non-keratinizing/undifferentiated subtypes, while in low-incidence areas, most cases belong to conventional keratinizing squamous cell carcinoma [1]. NPC is multifactorial, with the most important risk factor being EBV infection, common in infants and young adults in endemic regions. Other risk factors include salt-preserved foods, tobacco and alcohol use, and lately anecdotal associations with HPV infection, especially in non-endemic regions [7, 8].
NPC does not have a specific clinical presentation; however, headache, facial numbness, neck mass, nasal obstruction, epistaxis, and otitis media, together with risk factors, should raise suspicion for NPC. Unfortunately, due to its location and vague clinical presentation, most patients remain asymptomatic and are diagnosed with locally advanced disease. NPC is diagnosed preferentially with an endoscope-guided biopsy of the primary tumor, followed by adequate staging and pretreatment plasma EBV-DNA levels [9, 10].
As most patients are diagnosed in locally advanced scenarios, the development of new therapeutics is essential. In this manuscript, we focus on the current landscape of treatment and multiple clinical trials where the latest advances have been made. Understanding state-of-the-art treatments for NPC in local and locally advanced diseases, how it has changed, and future investigations are crucial.
Treatment for local disease
Local disease, classified as early stage I disease, encompasses patients with a tumor limited to the nasopharynx, or adjacent oropharynx, nasal cavity, without parapharyngeal involvement (T1), and no lymph node (N0) or distant metastasis (M0). Treatment at this stage has had limited advances regarding management, as international consensus establishes treatment with radiation therapy (RT) alone, as they are radiosensitive, have a limited surgical approach, and RT achieves excellent local control [11]. Precisely, intensity modulated RT (IMRT) is the mainstay treatment at this stage.
IMRT
Radiotherapy techniques have evolved, with IMRT demonstrating better results than 2D RT (two-dimensional radiotherapy) and 3D RT (three-dimensional radiotherapy). IMRT delivers high doses of radiation to the tumor, reduces toxicity to surrounding healthy tissue, and enables dose escalation [12]. A retrospective study demonstrated a 5-year distant failure-free rate of 85% for IMRT compared to 81% for 3D RT and 78% for 2D RT, as well as a decreased toxicity rate (1.8% vs 3.5% vs 7.4%) [13]. IMRT also improves the quality of life and spares parotid involvement in the early stages compared to conventional radiotherapy [14]. A phase II trial by Lee et al. evaluated the efficacy of IMRT in locoregionally advanced disease, including nine patients with stage I disease treated with IMRT only [15]. For these nine patients, none of them had a locoregional failure at a median follow-up of 2.6 years. This established IMRT alone as the standard of care for stage I NPC, providing the longest local recurrence-free survival.
Is there a role for surgery?
Surgical approaches are not the mainstay initial treatment for early disease, due to complex anatomical locations, access, and neurovascular damage. Surgery may be indicated in residual node disease post-radiation therapy and isolated neck recurrence. Its use as a first-line treatment over IMRT is not recommended. Liu et al. compared the use of IMRT vs minimally invasive surgery via an endoscopic nasopharyngoscopy in 339 patients with stage I NPC, of whom only 10 underwent surgical approach [16]. The study demonstrated similar survival outcomes with a 100% 5-year overall survival (OS), local relapse-free survival, regional relapse-free survival, and distant metastasis-free survival (DMFS), compared to 99.1%, 97.7%, 99.0%, and 97.4%, respectively for those treated with IMRT. The major emphasis of the study was regarding the impact on quality of life and lower medical costs for those treated with the minimally invasive surgical approach. This study offers the minimally invasive surgical approach as an alternative strategy, especially for patients who refuse IMRT due to toxicity concerns. However, performing a surgical approach over IMRT must be discussed with a multidisciplinary team.
Treatment for locally advanced disease
Locally advanced scenarios (stage III to IVA) together with metastatic scenarios (IVB) are the most common stages at which patients with NPC are diagnosed. Patients with the locally advanced disease include those cases involving at least one lymph node or bilateral retropharyngeal nodes (N1) and/or tumor extension into the parapharyngeal space (T2), bony structures (T3), or intracranial extensions (T4) but have not metastasized distantly (M0). Stage II (T1/T2, N1 or T2 N0), also known as intermediate disease, is not considered an advanced scenario, and treatment is based on RT with or without concurrent chemotherapy depending on the risk of disease recurrence.
Role of RT
IMRT must be the preferred mode, as mentioned previously. However, for locally advanced diseases, RT alone is not an option, as treatment must include concurrent chemoradiotherapy (CCRT). Important changes that have been done in RT for NPC compared to the past include potentially avoiding bilateral whole-neck RT where selective neck radiation in the node-negative neck can be offered, which is limited to retropharyngeal lymph nodes and levels II, III, and VA [17]. The reasoning for sparing nodes in the lower neck and IB level comes from the common pathway of lymphatic spread, with retropharyngeal and level II neck nodes being the most commonly affected. Avoiding RT at these nodes, in particular IB, is useful as it reduces xerostomia. IMRT has been demonstrated to achieve a 5-year local control of 90% for T3 disease and 74–80% for T4 [18, 19].
Future advances in the area of radiotherapy include accurate methods to delineate gross tumor volume, appropriate identification of margins, and clinical target volume, making these characteristics the objective of precision RT which is being accomplished by computer tomography, nowadays known as image-guided RT. These advances have the goal of limiting tissue damage and accurately identifying the radiated area [11].
Concurrent chemoradiation (CRT)
Concurrent CRT is recognized as the backbone for treating locally advanced NPC as established by the phase III United States Intergroup 0099 trial led by Al-Sarraf et al., where patients received chemoradiation with cisplatin followed by adjuvant chemotherapy (AC) with cisplatin plus fluorouracil or RT alone [20]. The median progression-free survival (PFS) at 15 months was not reached for the chemoradiotherapy group compared to the RT alone group, and the 3-year PFS rate was 69% vs 24% respectively. Similar results were observed for OS, in which the median OS was not reached for the CRT group and the 3-year survival rate was 78% vs 47%, respectively. Multiple clinical trials have demonstrated the benefit of concurrent CRT vs RT alone for both 5-year OS and 5-year PFS [15, 21,22,23]. Taking these studies into consideration, the 5-year OS and 5-year PFS were 75.3% and 65.9%, respectively for concurrent CRT vs 64.1% and 53.7% for those with RT alone. A meta-analysis of 4798 patients in which most of whom were stage III-IVB, an absolute OS benefit of 6% was observed at 5 years and 8% at 10 years [24]. Another meta-analysis focused on endemics demonstrated a benefit for concurrent CRT at 2, 3, and 5 years for OS, confirming the superiority of concurrent CRT over RT [25]. Similarly, a meta-analysis performed in Chinese patients proved that concurrent CRT improved the overall response rate, complete response rate, and had longer OS compared to RT; however, concurrent CRT had a higher degree of hematologic toxicities and mucositis [26]. On the other hand, a meta-analysis in non-endemic populations found that concurrent CRT improved both OS (10-year OS 59% vs 51%) and PFS (10-year PFS 52% vs 44%) [27].
While it is well established that the backbone treatment for locally advanced NPC is CRT, questions regarding the addition of induction chemotherapy (IC) or adjuvant chemotherapy (AC) are the main objective of the research, coupled with the role of targeted therapies, predictive biomarkers, and reducing toxicity [28, 29].
Adjuvant (AC) vs induction chemotherapy (IC). Which one is better?
The question regarding AC vs IC is the most debatable and studied aspect of the treatment of locoregionally advanced NPC. AC has been recognized as the standard of care since 1998 when cisplatin plus fluorouracil was the recommended regimen [20]. Additional trials validated these findings [30, 31]. One clinical trial confirmed these results, demonstrating a benefit for 2- and 3-year OS rates in favor of AC [30]. Similarly, control trials in endemic and non-endemic regions proved the survival benefit of AC with no increase in late toxicities [31,32,33]. A meta-analysis demonstrated that concurrent CRT followed by AC improved 10-year OS by 14% [27]. Prognostic and predictive factors such as EBV-DNA load are studied. Twu et al. demonstrated that AC reduced distant failure and improved OS in patients with persistently detectable EBV-DNA levels after concurrent CRT. Patients with persistent detectable EBV-DNA levels experience a lower tumor relapse with AC (45.5%) vs those without (71.2%) [34].
Studies have also demonstrated the opposite results. A clinical trial comparing RT alone vs AC (vincristine, cyclophosphamide, and doxorubicin) demonstrated no significant differences at 48 months in terms of relapse-free survival and OS [35]. Later on, a phase III study demonstrated no significant benefit for OS (60.5% RT vs 54.5% AC) and relapse-free survival (49.5% RT vs 54.4% AC) [36]. A recent phase III multicenter trial in the Chinese population comparing concurrent CRT + AC (cisplatin and fluorouracil) vs concurrent CRT alone demonstrated at 37.8 months and 68.4 months no significant survival benefit or difference regarding toxicities [37]. Similarly, a meta-analysis demonstrated no significant differences between concurrent CRT plus AC and concurrent CRT alone for all endpoints [38].
IC may be recommended for patients with locally advanced disease. The first study to report a benefit in OS reported a 26.5% improvement in 3-year OS by adding cisplatin + docetaxel [39]. Research comparing IC followed by concurrent CRT vs concurrent CRT alone is vast, with trials demonstrating a benefit for IC, including a phase III trial in locoregionally advanced EBV + NPC comparing gemcitabine + cisplatin + CRT vs concurrent CRT alone [40]. At a median follow-up of 42.7 months, a benefit in both 3-year recurrence-free survival (85.3% vs 76.5%) and 3-year OS (94.6% vs 90.3%) in favor of IC was reported [40]. Additionally, the IC reported a 9.2% incidence of grade 34 toxicity compared to 11.4% for concurrent CRT. The follow-up study at 70 months reported an improved 5-year OS (88% vs 79%), failure-free survival (FFS) (81% vs 67%), DMFS (90% vs 78%), and locoregional free survival (88% vs 83%) in favor of IC [41]. This study highlights the importance of Epstein-Barr virus (EBV) DNA load for guiding individualized treatment, in which patients with DNA loads < 4000 copies/mL do not benefit from IC compared to those with a higher viral load. Mané et al.’s meta-analyses identified that the addition of IC to concurrent CRT provides a significant benefit in OS and PFS compared to concurrent CRT alone [42].
Few studies have directly compared IC vs AC, with conflicting evidence being reported. One study demonstrated IC improves OS, PFS, DMFS, and locoregional recurrence-free survival [43]. On the other hand, another meta-analysis demonstrated that AC achieves a higher survival benefit, but IC had greater distant control [44]. A propensity score-matched analysis demonstrated IC + concurrent CRT improved survival in T3 and N2 disease, with AC improving local control but not survival outcomes in T4 disease [45].
It is clear that more work is needed to determine if there is a benefit in favor of IC based on the conflicting evidence. On one hand, IC could offer better tolerability, easier delivery, and overcome poor tolerance, but IC could affect the definitive CRT dose. Based on current evidence, concurrent CRT with cisplatin with either adjuvant or induction chemotherapy is the treatment of choice. The NRG HN001 trial evaluates the non-inferiority of the omission of AC in low-risk, locally advanced NPC patients.
EBV-DNA levels have been demonstrated to be a determining factor in both IC (favoring those with high viral loads) and identifying patients in need of AC (favoring those with persistently elevated levels). A clinical trial directly comparing them could be difficult to perform; therefore, a direct comparison should focus on answering a specific outcome and future trials in studying the role of EBV-DNA levels in those in need of AC.
The following table (Table 1) summarizes the most important clinical trials highlighting the benefit of concurrent CRT, AC, and IC and their impact on patient survival.
What is the best chemotherapy regimen?
Concurrent CRT
Cisplatin is still the recommended agent of choice for concurrent CRT [20, 52, 53]. Studies focus on analyzing the efficacy and toxicity regarding the administration method as weekly or bolus dosing. A phase II trial compared weekly vs triweekly cisplatin therapy concurrently with RT and demonstrated that weekly cisplatin improved quality of life with no differences regarding 3-year PFS (64.9% vs 63.8%) or grade 3–4 toxicities (47.2% vs 39.3%) [54]. A separate phase III trial compared bolus cisplatin therapy (once every 3 weeks) vs weekly cisplatin concurrently with IMRT. There was a benefit regarding lower toxicities (55.8% vs 66.3%), especially hematologic and auditory, in favor of bolus cisplatin, with no differences in 3-year FFS (85% vs 86%) [52]. It is important to have alternatives for platinum-ineligible patients (e.g., renal failure), but unfortunately, few studies exist. The two alternative options that have been studied include carboplatin and oxaliplatin. Carboplatin may be an alternative due to its lower toxicity and similar efficacy, as demonstrated by a phase III trial in which carboplatin demonstrated a benefit regarding completion of concurrent CRT (73% vs 53%), a higher percentage of completed AC (70% vs 42%), similar 3-year PFS (60.9% carboplatin vs 63.9% cisplatin), and 3-year OS (79.2% carboplatin vs 77.7% cisplatin) [55]. Another trial compared RT alone vs concurrent CRT with oxaliplatin and demonstrated a benefit on 5-year OS (60.2% vs 73.2%), metastasis-free survival (63.0% vs 74.7%), and similar toxicities in favor of oxaliplatin [21]. Nevertheless, no study has compared oxaliplatin vs cisplatin directly.
Adjuvant chemotherapy
Cisplatin plus fluorouracil has been the regimen of choice for AC [20, 31, 33]. However, other agents have demonstrated similar efficacy and lower toxicity and are considered part of clinical practice such as carboplatin plus fluorouracil [55]. The use of capecitabine as an AC to concurrent CRT has been studied in metronomic doses. This phase III trial demonstrated an improvement in 3-year FFS (85% vs 76%) and 3-year OS (93% vs 89%) in patients with high-risk locoregionally advanced NPC in favor of adjuvant metronomic capecitabine [56]. This study was performed in the Chinese population, and most patients received IC (docetaxel plus cisplatin). Adjuvant capecitabine has also been studied as a standard dose in stage III-IVB patients with at least one high-risk feature (T3-4 plus N2, any T stage plus N3, pretreatment EBV-DNA ≥ 20,000 copies/mL, tumor volume > 30 cm2, PET-CT uptake > 10, or multiple neck node metastases) [57]. At 44.8 months, adjuvant capecitabine demonstrated a benefit in 3-year FFS (87.7% vs 73.3%) and superior disease control over concurrent CRT alone. These two trials demonstrated higher grade 3–4 toxicities compared to concurrent CRT alone, but high compliance rates.
Induction chemotherapy
The standard regimen for IC consists of gemcitabine plus cisplatin [40, 41]. Nevertheless, different chemotherapeutic regimens have been studied. A phase III trial compared the use of IC based on cisplatin + fluorouracil + docetaxel (TPF) plus concurrent CRT vs concurrent CRT alone [58]. The addition of TPF to concurrent CRT improved 3-year FFS by 8% more than concurrent CRT alone with acceptable toxicity and improved 3-year OS (92% vs 86%). Yang et al. reported their 5-year follow-up results comparing IC with fluorouracil plus cisplatin followed by concurrent CRT vs concurrent CRT alone [59]. At 83 months, a benefit was observed in 5-year disease-free survival (DFS) (73.4% vs 63.1%), DMFS (82.8% vs 73.1%), and 5-year OS (80.8% vs 76.8%) in favor of IC, with no difference in grade ≥ 3 toxicities. Similarly, a phase III multicenter study comparing IC with cisplatin and fluorouracil vs concurrent CRT alone demonstrated a benefit regarding DFS but higher grade 3–4 toxicity [60]. These two studies provide regimens such as TPF and fluorouracil plus cisplatin as alternative options. A phase III GORTEC trial including patients’ stages III–IVB proved the addition of TPF as an induction regimen plus concurrent CRT improved 3-year PFS [61]. Few studies have directly compared different regimen efficacy. Published this year at JAMA Oncology, a phase III trial compared the use of cisplatin + paclitaxel + capecitabine (TPC) vs cisplatin + fluorouracil as IC followed by concurrent CRT [62]. TPC improved FFS (84% vs 69%), DMFS (91% vs 80%), and locoregional relapse-free survival (94% vs 87%) was well tolerable compared to cisplatin plus fluorouracil. Similarly, a recently published trial compared IC with lobaplatin and fluorouracil vs cisplatin and fluorouracil followed by concurrent CRT demonstrating that lobaplatin with fluorouracil resulted in non-inferior survival with fewer toxicity [63]. Even though gemcitabine + cisplatin is considered the preferred regimen, TPC and lobaplatin are acceptable alternatives for this population.
The role of EBV in therapeutics
The prognosis for local disease patients is promising, with a 5-year OS of 93.2% and an 8-year OS of 85.5% [64]. Retrospective studies have established a 5-year disease-specific survival rate of 94–97% with IMRT alone [65]. The role of pretreatment EBV-DNA levels as prognostic factors impacting the 5-year survival rate in the early stage is well established, with a cut-off point of < 4000 copies/mL having a 91% survival rate and ≥ 4000 copies/mL having a 64% survival rate [66]. These also apply to stage II disease. The role of post-treatment EBV-DNA levels is not well established; however, it may have a role in predicting distant metastasis and stratifying patients for adjuvant therapy or follow-up [67]. Unfortunately, these studies are focused on locally advanced disease (II-IVB), with limited studies specifying the role of post-radiation EBV-DNA levels in patients treated with IMRT alone. One study evaluated the use of post-treatment levels in 385 patients treated with IMRT, of whom 23 patients were stage I [68]. They showed that detectable plasma EBV-DNA post-treatment could be found in patients with undetectable pretreatment levels and was significantly associated with tumor recurrence, especially distant metastasis, regardless of EBV-DNA level status pretreatment.
Plasma EBV-DNA is the most significant prognostic biomarker used to identify patients at risk of failure post-concurrent CRT which are those who should receive AC [69]. Twu et al. demonstrated that adjuvant tegafur-uracil improved distant control and OS in patients with persistently detectable EBV-DNA post-RT vs those without AC [34]. One trial measuring plasma EBV-DNA levels at 6–8 weeks post-RT demonstrated that post-RT plasma levels correlate with locoregional failure, distant metastasis, and death; however, the use of AC did not improve relapse-free survival [70]. Even though a benefit was not observed, failure could have been influenced by the 6-week cut-off point. The previous hypothesis is based on patients having varying periods of time with detectable EBV-DNA plasma levels; one study demonstrated a detectable decreasing rate of 11.5% between 8 and 16 weeks post-RT [71]. The optimal timing to evaluate post-treatment plasma EBV-DNA remains to be identified. Post-RT plasma EBV-DNA can work as an early surrogate maker for long-term survival; patients with detectable levels who experienced subsequent clearance had a greater survival compared to patients with initially undetectable post-RT plasma levels [72]. New technologies for measuring circulating cell-free EBV-DNA (cfEBV DNA) via liquid biopsy have had promising results. A study measuring cfEBV DNA at 3 months post-RT and every 3 to 12 months demonstrated that patients with detectable cfEBV DNA had a higher recurrence rate compared to those with undetectable levels (63.8% vs 8.6%), with high sensitivity, specificity, and accuracy for local, regional recurrence, and distant metastasis [73]. Interestingly, patients with disease recurrence had detectable cfEBV DNA levels 2.3 months prior to clinical and/or radiological recurrence. The ongoing NRG HN001 trial is evaluating individualized treatment based on EBV-DNA. (NCT02135042).
New systemic and targeted therapies
One of the main areas of research is reducing toxicities via new therapeutic options and identifying prognostic factors such as plasma EBV-DNA.
Targeted therapies including growth factor receptor inhibitors, vascular endothelial growth factor inhibitors, and immunotherapies have completely changed the management of patients with lung, head and neck, and renal cancer. Therefore, studies have evaluated these agents in NPC with most studies focusing on China. A phase II study evaluated the use of concurrent cetuximab-cisplatin and IMRT in locoregionally advanced non-keratinizing NPC demonstrating that its weekly use is a feasible strategy with manageable toxicities and an 86.5% 2-year PFS [74]. Niu et al. evaluated the use of weekly cetuximab plus IMRT with or without chemotherapy and demonstrated it to be effective with a 3-year local failure-free survival (LFFS), region failure-free survival (RFFS), DMFS, PFS, and OS of 86.3%, 83.4%, 83.6%, 70.5%, and 90.9%, respectively [75]. Weekly cetuximab with AC followed by IMRT resulted in 100% local and regional control and 95.2% distant control [76]. Bevacizumab, an antiangiogenic agent, in combination with standard chemoradiation, demonstrated to be a feasible alternative for delaying the progression of subclinical distant disease, achieving 83.7% 2-year locoregional control, 90% DMFS, 74.7% PFS, and 90.9% OS [77]. Results of nimotuzumab (anti-EGF monoclonal antibody) plus chemoradiotherapy vs chemoradiotherapy alone were presented at ASCO 2022, reporting a 5-year OS increase in favor of nimotuzumab (76.9% vs 64.3%) and a good safety profile [78]. Unfortunately, PD1/PDL1 inhibitors are neither standard predictive biomarkers nor immunotherapeutic agents for the locoregional disease, and their use cannot be extrapolated as for other cancers. However, there are two ongoing clinical trials in locoregional NPC analyzing a locally manufactured PD1 monoclonal antibody in China (camrelizumab) and nivolumab (NCT03427827 and NCT03267498).
The multiple ongoing advanced clinical trials will provide more information in the future regarding the best treatment for patients (Table 2).
Role and impact of viral positivity and targeted therapy
Epstein-Barr virus (EBV)
Worldwide EBV infection is common, with primary infection occurring in childhood. With NPC being a rare cancer and EBV being a common infection, additional cofactors such as the age of infection and genetic variations most likely mediate the risk of EBV in NPC. A younger age of EBV infection has been suggested as a contributing factor. A Swedish study demonstrated via indicators of early-life EBV infection an association between earlier infection and the development of NPC and an inverse association with infectious mononucleosis [79]. A study in the Chinese population identified four high-risk EBV variant mutations (BALF2, BNFR1, V12221, and RPMS1) and their interaction with the immune system as contributing to an increased risk of NPC [80, 81]. These high-risk EBV variants affect the host immune response, promoting an IgA-dependent response. With these antibodies being elevated in NPC patients, they work as the basis for screening high-risk populations and are useful prognostic indicators [82].
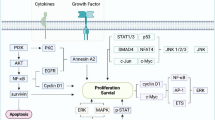
The vast analysis of EBV has offered the opportunity to develop new therapeutic advances focused on targeting EBNA1 and LMP1. EBNA1 plays an important role in viral DNA maintenance, survival, and oncogenic transformation of host cells [83]. Preclinical studies have studied mechanisms to disrupt EBNA1 function and avoid EBV latency including blockage of DNA binding/dimerization, disruption of the dimer-dimer interface, and targeting the trimer interface [84]. Latent membrane lipoproteins (LMPs), especially LMP1, are viral oncoproteins that promote cell proliferation, survival, angiogenesis, and immunosuppressive effects against cancer cells [85]. Inhibition of LMP1 in NPC cells demonstrated promising results in inhibiting cell proliferation, inducing apoptosis, and suppressing tumor growth [86]. A trial evaluated the efficacy of targeting LMP1 via a DNAzyme intratumorally in conjunction with radiotherapy [87]. The study demonstrated a higher mean tumor regression rate at 12 weeks in those treated with DNAzyme, an impact on tumor microvascular permeability, and a higher number of samples with undetectable EBV-DNA levels. Other strategies targeting LMP1 include infusion with autologous EBV-specific cytotoxic lymphocytes, LMP1-specific antibodies, and peptide-based inhibitors [88]. Finally, switching the EBV latency state to a lytic cycle promotes cell death and an immune response against EBV-positive cancer cells. This switch can be performed by multiple agents including chemotherapeutic agents, histone deacetylase inhibitors, protein kinase C activators, proteasome inhibitors, and antibacterial agents [84].
EBV analysis may offer a personalized treatment by prognosticating which patients benefit from AC.
Human papilloma virus (HPV)
EBV is the best-known causative agent in NPC; however, reports have found an association with HPV in certain subgroups. This hypothesis is based on the important tumorigenesis role that HPV plays in head and neck cancers.
NPC WHO types II and III have a strong association with EBV + , but it is less established for WHO type I. A cancer center demonstrated an association with oncogenic HPV in WHO type I NPC [89]. On the other hand, a study by the American Cancer Society associated with NPC WHO type III in Southern China demonstrated 91.9% EBV + , 7.7% HPV + , and 0.6% coinfection. The interesting results were that EBV − /HPV + patients had a lower local recurrence rate compared to EBV + /HPV − (6.4% vs 13.8%), as well as a longer 5-year disease-free survival (89.8% vs 70.8%) and 5-year OS (86% vs 72%) [90]. Different results have been found in non-endemic populations, where HPV + has been correlated with WHO grade II tumors in Caucasian patients and worse outcomes compared to EBV + tumors [91]. These two studies raise awareness that high-risk HPV may play a role in the development of NPC and that prognosis may differ between populations. Unfortunately, the evidence is limited and has conflicting results. For example, one study in the UK found eleven cases with concurrent overexpression of HPV p16, with most cases occurring in white patients with non-keratinizing carcinomas, and none of the HPV + cases showing coinfection with EBV and no OS difference [92]. Similarly, a study in Japan demonstrated a limited number of HPV + NPC type I cases with no impact on survival and concluded no significant role in carcinogenesis [93].
The evidence regarding the role of HPV in NPC carcinogenesis and its clinical significance is limited. So far, it can be assumed that HPV’s role may differ between populations; it is not well established in which WHO histologic type is more prominent, and its presence in the absence of EBV + strongly suggests HPV as a causal factor in NPC development.
Molecular biology in nasopharyngeal carcinoma
Germline mutations
Most cases of NPC occur sporadically; therefore, information and identification of germline mutations are limited. Certain germline variants identified in familial NPC cases promote tumor progression and increase the risk of additional somatic mutations. Even though NPC exhibits significant familial aggregation, the susceptible genes are not well identified. CYLD somatic mutations play an important role, but germline mutations are associated with familial cylindromatosis characterized by multiple benign head and neck tumors but not NPC [94].
Most of the germline mutations play a role in DNA damage repair mechanisms including BRCA1/2, PRKDC, MLH1, and KMT2C, host defense mechanisms genes (MST1R), virus infectivity, BCL2L12, NEDD4L, and signaling pathways (NOTCH and DLL3) [95,96,97]. A study analyzed via whole-exome sequencing 13 NPC germline mutations. Missense mutations in the POLN gene, P577L, R303Q, and F545C were associated with familial NPC risk, especially EBV + [98]. This occurs because the POLN gene is involved in the lytic replication of EBV in NPC cells, promoting viral DNA replication and proliferation. Whole genome sequencing has identified various germline variants that could predispose to NPC which include MST1R, NIPAL1, ITGB6, notch signaling, and pathogenic variants in JAK2, PRDM16, LRP1B, NIN, NKX2-1, and FANCE [99, 100]. The latter genes were associated to intervene in endocytosis and immune-modulating pathways, indicating an important role for immunotherapeutic target drugs. Similarly, multiple germline mutations were identified in EBV + NPC patients, mainly missense and silent mutations in chromosomes 1, 12, and 17 (GON4L, KRAS, MTOR, NBPF10, NOTCH2, ZC3H11A, and KRTAP1-5) [94]. Additional studies have identified the role of the MHC1 complex including NLCR5, HLA-A/B/C impacting NPC, and its critical role in EBV antigenic presentation [101]. Studying these specific germline mutations can help understand NPC pathogenesis and diagnose patients earlier.
Somatic mutations
Because germline mutations are important for family considerations in both local and metastatic settings, we will cover them in this article. Somatic mutations and association with targeted therapies will be covered in the metastatic NPC article.
Conclusion
Current research and the latest treatment advances in locally advanced NPC include induction vs adjuvant chemotherapy, which chemotherapeutic regimen, and the role of de-escalation. Research focusing on the role of EBV may guide aggressive treatment in those with elevated pretreatment and persistent EBV levels following treatment. With the important role of molecular biology, new and targeted therapies may offer personalized treatment. Similar to other cancers, somatic and germline mutations play an important role in NPC oncogenesis. The analysis of these mutations offers a new method of identifying familial cases earlier. Additionally, in the locally advanced setting studies, have been initiated evaluating VEGF, EGFR, and checkpoint inhibitors added to chemoradiation. We await the outcome.
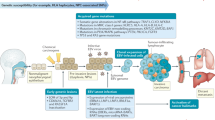
This review has the goal of offering a comprehensive algorithm (Fig. 1) summarizing the management of patients with locally advanced NPC. Details regarding these treatments can be found in the specific sections of the manuscript.
References and Recommended Reading
Chang ET, Ye W, Zeng YX, Adami HO. The evolving epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2021;30(6):1035–47.
Global Cancer Observatory. International Agency for Research on Cancer. 2022. https://gco.iarc.fr/. Accessed 15 Feb 2023.
Ferlay J, Ervik M, Lam F, Colombet M, Mery L PM. Global cancer observatory: cancer today. [Internet]. International Agency for Research on Cancer (IARC). 2018. Available from: https://gco.iarc.fr/today. Accessed 15 Feb 2023.
Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765–77.
Rueda Domínguez A, Cirauqui B, García Castaño A, Alvarez Cabellos R, Carral Maseda A, Castelo Fernández B, et al. SEOM-TTCC clinical guideline in nasopharynx cancer (2021). Clin Transl Oncol. 2022;24(4):670–80. https://doi.org/10.1007/s12094-022-02814-x.
Stelow EB, Wenig BM. Update from the 4th edition of the World Health Organization classification of head and neck tumours: nasopharynx. Head Neck Pathol. 2017;11(1):16–22.
Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. 2016;387(10022):1012–24. https://doi.org/10.1016/S0140-6736(15)00055-0.
Dogan S, Hedberg ML, Ferris RL, Rath TJ, Assaad AM, Chiosea SI. Human papillomavirus and Epstein-Barr virus in nasopharyngeal carcinoma in a low-incidence population. Head Neck [Internet]. 2014;36(4):511–6. https://doi.org/10.1002/hed.2331.
Bossi P, Chan AT, Licitra L, Trama A, Orlandi E, Hui EP, et al. Nasopharyngeal carcinoma: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(4):452–65. https://doi.org/10.1016/j.annonc.2020.12.007.
Lee VHF, Kwong DLW, Leung TW, Choi CW, O’Sullivan B, Lam KO, et al. The addition of pretreatment plasma Epstein-Barr virus DNA into the eighth edition of nasopharyngeal cancer TNM stage classification. Int J Cancer. 2019;144(7):1713–22.
Lee AWM, Ma BBY, Ng WT, Chan ATC. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. 2015;33(29):3356–64.
Taylor A, Powell MEB. Intensity-modulated radiotherapy - what is it? Cancer Imaging. 2004;4(2):68–73.
Lee AWM, Ng WT, Chan LLK, Hung WM, Chan CCC, Sze HCK, et al. Evolution of treatment for nasopharyngeal cancer - success and setback in the intensity-modulated radiotherapy era. Radiother Oncol. 2014;110(3):377–84. https://doi.org/10.1016/j.radonc.2014.02.003.
Kam MKM, Leung SF, Zee B, Chau RMC, Suen JJS, Mo F, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25(31):4873–9.
Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol. 2009;27(22):3684–90.
Liu YP, Lv X, Zou X, Hua YJ, You R, Yang Q, et al. Minimally invasive surgery alone compared with intensity-modulated radiotherapy for primary stage i nasopharyngeal carcinoma. Cancer Commun. 2019;39(1):1–11. https://doi.org/10.1186/s40880-019-0415-3.
Li JG, Yuan X, Zhang LL, Tang YQ, Liu L, Chen XD, et al. A randomized clinical trial comparing prophylactic upper versus whole-neck irradiation in the treatment of patients with node-negative nasopharyngeal carcinoma. Cancer. 2013;119(17):3170–6.
Wu F, Wang R, Lu H, Wei B, Feng G, Li G, et al. Concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: treatment outcomes of a prospective multicentric clinical study. Radiother Oncol. 2014;112(1):106–11. https://doi.org/10.1016/j.radonc.2014.05.005.
Yi J, Huang X, Gao L, Luo J, Zhang S, Wang K, et al. Intensity-modulated radiotherapy with simultaneous integrated boost for locoregionally advanced nasopharyngeal carcinoma. Radiat Oncol. 2014;9(1):2–8.
Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol [Internet]. 1998;16(4):1310–7. https://doi.org/10.1200/JCO.1998.16.4.1310.
Wu X, Huang PY, Peng PJ, Lu LX, Han F, Wu SX, et al. Long-term follow-up of a phase III study comparing radiotherapy with or without weekly oxaliplatin for locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. 2013;24(8):2131–6.
Lee AWM, Tung SY, Chua DTT, Ngan RKC, Chappell R, Tung R, et al. Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. 2010;102(15):1188–98.
Zhang L, Zhao C, Peng PJ, Lu LX, Huang PY, Han F, et al. Phase III study comparing standard radiotherapy with or without weekly oxaliplatin in treatment of locoregionally advanced nasopharyngeal carcinoma: Preliminary results. J Clin Oncol. 2005;23(33):8461–8.
Blanchard P, Lee AWM, Carmel A, Wai Tong N, Ma J, Chan ATC, et al. Meta-analysis of chemotherapy in nasopharynx carcinoma (MAC-NPC): an update on 26 trials and 7080 patients. Clin Transl Radiat Oncol. 2021;2022(32):59–68.
Zhang L, Zhao C, Ghimire B, Hong MH, Liu Q, Zhang Y, et al. The role of concurrent chemoradiotherapy in the treatment of locoregionally advanced nasopharyngeal carcinoma among endemic population: a meta-analysis of the phase iii randomized trials. BMC Cancer. 2010;10(1):558. https://doi.org/10.1186/1471-2407-10-558.
He Y, Guo T, Guan H, Wang J, Sun Y, Peng X. Concurrent chemoradiotherapy versus radiotherapy alone for locoregionally advanced nasopharyngeal carcinoma in the era of intensitymodulated radiotherapy: a meta-analysis. Cancer Manag Res. 2018;10:1419–28.
Blanchard P, Lee A, Marguet S, Leclercq J, Ng WT, Ma J, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16(6):645–55. https://doi.org/10.1016/S1470-2045(15)70126-9.
Hui EP, Ma BBY, Chan ATC. The emerging data on choice of optimal therapy for locally advanced nasopharyngeal carcinoma. Curr Opin Oncol. 2020;32(3):187–95.
Ma BBY, Chen Y-P, Hui EP, Liu X, Chan AKC, Chan ATC, et al. Recent advances in the development of biomarkers and chemoradiotherapeutic approaches for nasopharyngeal carcinoma. Am Soc Clin Oncol Educ B. 2020;40:270–80.
Wee J, Tan EH, Tai BC, Wong HB, Leong SS, Tan T, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union Against Cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol. 2005;23(27):6730–8.
Lee AWM, Tung SY, Chan ATC, Chappell R, Fu YT, Lu TX, et al. A randomized trial on addition of concurrent-adjuvant chemotherapy and/or accelerated fractionation for locally-advanced nasopharyngeal carcinoma. Radiother Oncol. 2011;98(1):15–22. https://doi.org/10.1016/j.radonc.2010.09.023.
Chen Y, Sun Y, Liang SB, Zong JF, Li WF, Chen M, et al. Progress report of a randomized trial comparing long-term survival and late toxicity of concurrent chemoradiotherapy with adjuvant chemotherapy versus radiotherapy alone in patients with stage III to IVB nasopharyngeal carcinoma from endemic regions of Ch. Cancer. 2013;119(12):2230–8.
Lee AWM, Tung SY, Ng WT, Lee V, Ngan RKC, Choi HCW, et al. A multicenter, phase 3, randomized trial of concurrent chemoradiotherapy plus adjuvant chemotherapy versus radiotherapy alone in patients with regionally advanced nasopharyngeal carcinoma: 10-year outcomes for efficacy and toxicity. Cancer. 2017;123(21):4147–57.
Twu CW, Wang WY, Chen CC, Liang KL, Jiang RS, Te WuC, et al. Metronomic adjuvant chemotherapy improves treatment outcome in nasopharyngeal carcinoma patients with postradiation persistently detectable plasma Epstein-Barr virus deoxyribonucleic acid. Int J Radiat Oncol Biol Phys. 2014;89(1):21–9. https://doi.org/10.1016/j.ijrobp.2014.01.052.
Rossi A, Molinari R, Boracchi P, Del Vecchio M, Marubini E, Nava M, et al. Adjuvant chemotherapy with vincristine, cyclophosphamide, and doxorubicin after radiotherapy in local-regional nasopharyngeal cancer: results of a 4-year multicenter randomized study. J Clin Oncol. 1988;6(9):1401–10.
Chi KH, Chang YC, Guo WY, Leung MJ, Shiau CY, Chen SY, et al. A phase III study of adjuvant chemotherapy in advanced nasopharyngeal carcinoma patients. Int J Radiat Oncol Biol Phys. 2002;52(5):1238–44.
Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y, et al. Adjuvant chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase 3 multicentre randomised controlled trial. Eur J Cancer. 2017;75(January):150–8. https://doi.org/10.1016/j.ejca.2017.01.002.
Fang L, Shi L, Wang W, Hu T, Rao X. Which treatment is better than concurrent chemoradiotherapy about survival for stage III or IV locally advanced nasopharyngeal carcinoma? An updated Bayesian network meta-analysis of randomized controlled trials. Eur Arch Oto-Rhino-Laryngol. 2021;278(10):3633–42. https://doi.org/10.1007/s00405-021-06614-x.
Hui EP, Ma BB, Leung SF, King AD, Mo F, Kam MK, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol. 2009;27(2):242–9.
Zhang Y, Chen L, Hu G-Q, Zhang N, Zhu X-D, Yang K-Y, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med. 2019;381(12):1124–35.
Zhang Y, Chen L, Hu G-Q, Zhang N, Zhu X-D, Yang K-Y. Final overall survival analysis of gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma: a multicenter, randomized phase III trial. J Clin Oncol. 2022;40(22):2420–5.
Mané M, Benkhaled S, Dragan T, Paesmans M, Beauvois S, Lalami Y, et al. Meta-analysis on induction chemotherapy in locally advanced nasopharyngeal carcinoma. Oncologist. 2021;26(1):e130–41.
Zhang B, Li MM, Chen WH, Zhao JF, Chen WQ, Dong YH, et al. Association of chemoradiotherapy regimens and survival among patients with nasopharyngeal carcinoma: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(10):1–20.
Ribassin-Majed L, Marguet S, Lee AWM, Ng WT, Ma J, Chan ATC, et al. What is the best treatment of locally advanced nasopharyngeal carcinoma? an individual patient data network meta-analysis. J Clin Oncol. 2017;35(5):498–505.
Tang SQ, Xu C, Wang XS, Tang LL, Li WF, Chen L, et al. Induction versus adjuvant chemotherapy combined with concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: a propensity score-matched analysis. Oral Oncol. 2020;105(January):104686. https://doi.org/10.1016/j.oraloncology.2020.104686.
Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wang WY. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. 2003;21(4):631–7.
Chan ATC, Teo PML, Ngan RK, Leung TW, Lau WH, Zee B, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol. 2002;20(8):2038–44.
Lee AWM, Lau WH, Tung SY, Chua DTT, Chappell R, Xu L, et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol. 2005;23(28):6966–75.
Chan ATC, Leung SF, Ngan RKC, Teo PML, Lau WH, Kwan WH, et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. 2005;97(7):536–9.
Fountzilas G, Ciuleanu E, Bobos M, Kalogera-fountzila A, Eleftheraki AG, Karayannopoulou G, et al. Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: a randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeC. Ann Oncol. 2012;23(2):427–35.
Chua DT, Sham JS, Choy D, Lorvidhaya V, Sumitsawan Y, Thongprasert S, et al. Preliminary report of the Asian-Oceanian Clinical Oncology Association randomized trial comparing cisplatin and epirubicin followed by radiotherapy versus radiotherapy alone in the treatment of patients with locoregionally advanced nasopharyngeal carcinom. Asian-Oceanian Clinical Oncology Association Nasopharynx Cancer Study Group. Cancer. 1998;83(11):2270–83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9840526. Accessed 15 Feb 2023.
Xia WX, Lv X, Liang H, Liu GY, Sun R, Zeng Q, et al. A randomized controlled trial comparing two different schedules for cisplatin treatment in patients with locoregionally advanced nasopharyngeal cancer. Clin Cancer Res. 2021;27(15):4168–94.
Zhu Q, Hu H, Tang LQ, You R, Zhao JJ, Weng DS, et al. Weekly versus triweekly cisplatin plus intensity-modulated radiotherapy in locally advanced nasopharyngeal carcinoma: a propensity score analysis with a large cohort. J Cancer. 2018;9(19):3447–55.
Lee JY, Sun JM, Oh DR, Lim SH, Goo J, Lee SH, et al. Comparison of weekly versus triweekly cisplatin delivered concurrently with radiation therapy in patients with locally advanced nasopharyngeal cancer: a multicenter randomized phase II trial (KCSG-HN10–02). Radiother Oncol. 2016;118(2):244–50. https://doi.org/10.1016/j.radonc.2015.11.030.
Chitapanarux I, Lorvidhaya V, Kamnerdsupaphon P, Sumitsawan Y, Tharavichitkul E, Sukthomya V, et al. Chemoradiation comparing cisplatin versus carboplatin in locally advanced nasopharyngeal cancer: randomised, non-inferiority, open trial. Eur J Cancer. 2007;43(9):1399–406.
Chen YP, Liu X, Zhou Q, Yang KY, Jin F, Zhu XD, et al. Metronomic capecitabine as adjuvant therapy in locoregionally advanced nasopharyngeal carcinoma: a multicentre, open-label, parallel-group, randomised, controlled, phase 3 trial. Lancet. 2021;398(10297):303–13. https://doi.org/10.1016/S0140-6736(21)01123-5.
Miao J, Wang L, Tan SH, Li J-G, Yi J, Zhang Y. Adjuvant capecitabine in locoregionally advanced nasopharyngeal carcinoma: a multicenter randomized controlled phase III trial. J Clin Oncol. 2021;39(15):6005–6005.
Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17(11):1509–20. https://doi.org/10.1016/S1470-2045(16)30410-7.
Yang Q, Cao SM, Guo L, Hua YJ, Huang PY, Zhang XL, et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicentre randomised controlled trial. Eur J Cancer. 2019;119:87–96. https://doi.org/10.1016/j.ejca.2019.07.007.
Cao SM, Yang Q, Guo L, Mai HQ, Mo HY, Cao KJ, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase III multicentre randomised controlled trial. Eur J Cancer. 2017;75:14–23. https://doi.org/10.1016/j.ejca.2016.12.039.
Frikha M, Auperin A, Tao Y, Elloumi F, Toumi N, Blanchard P, et al. A randomized trial of induction docetaxel-cisplatin- 5FU followed by concomitant cisplatin-RT versus concomitant cisplatin-RT in nasopharyngeal carcinoma (GORTEC 2006–02). Ann Oncol. 2018;29(3):731–6.
Li W, Lv XHD. Effect of induction chemotherapy with paclitaxel, cisplatin, and capecitabine vs cisplatin and fluorouracil on failure-free survival for patients with stage IVA to IVB nasopharyngeal carcinoma: a multicenter phase 3 randomized clinical trial. JAMA Oncol. 2022;8(5):706–14.
Lv X, Cao X, Xia WX, Liu KY, Qiang MY, Guo L, et al. Induction chemotherapy with lobaplatin and fluorouracil versus cisplatin and fluorouracil followed by chemoradiotherapy in patients with stage III–IVB nasopharyngeal carcinoma: an open-label, non-inferiority, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22(5):716–26. https://doi.org/10.1016/S1470-2045(21)00075-9.
Au KH, Ngan RKC, Ng AWY, Poon DMC, Ng WT, Yuen KT, et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: a report of 3328 patients (HKNPCSG 1301 study). Oral Oncol. 2017;2018(77):16–21.
Su SF, Han F, Zhao C, Chen CY, Xiao WW, Li JX, et al. Long-term outcomes of early-stage nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy alone. Int J Radiat Oncol Biol Phys. 2012;82(1):327–33.
Leung SF, Zee B, Ma BB, Hui EP, Mo F, Lai M, et al. Plasma Epstein-Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol. 2006;24(34):5414–8.
Hui EP, Li WF, Ma BB, Lam WKJ, Chan KCA, Mo F, et al. Integrating postradiotherapy plasma Epstein-Barr virus DNA and TNM stage for risk stratification of nasopharyngeal carcinoma to adjuvant therapy. Ann Oncol. 2020;31(6):769–79.
Li W-F, Zhang Y, Huang X-B, Du X-J, Tang L-L, Chen L, et al. Prognostic value of plasma Epstein-Barr virus DNA level during posttreatment follow-up in the patients with nasopharyngeal carcinoma having undergone intensity-modulated radiotherapy. Chin J Cancer. 2017;36(1):1–9.
Hui EP, Ma BBY, Chan KCA, Chan CML, Wong CSC, To KF, et al. Clinical utility of plasma Epstein-Barr virus DNA and ERCC1 single nucleotide polymorphism in nasopharyngeal carcinoma. Cancer. 2015;121(16):2720–9.
Chan ATC, Hui EP, Ngan RKC, Tung SY, Cheng ACK, Ng WT, et al. Analysis of plasma Epstein-Barr virus DNA in nasopharyngeal cancer after chemoradiation to identify high-risk patients for adjuvant chemotherapy: a randomized controlled trial. J Clin Oncol. 2018;36(31):3091–100.
Lee VHF, Kwong DLW, Leung TW, Choi CW, Lai V, Ng L, et al. Prognostication of serial post-intensity-modulated radiation therapy undetectable plasma EBV DNA for nasopharyngeal carcinoma. Oncotarget. 2017;8(3):5292–308.
Hui EP, Ma BBY, Jacky Lam WK, Allen Chan KC, Mo F, Hemis Ai QY, et al. Dynamic changes of post-radiotherapy plasma Epstein-Barr virus DNA in a randomized trial of adjuvant chemotherapy versus observation in nasopharyngeal cancer. Clin Cancer Res. 2021;27(10):2827–36.
Chen FP, Huang XD, Lv JW, Wen DW, Zhou GQ, Lin L, et al. Prognostic potential of liquid biopsy tracking in the posttreatment surveillance of patients with nonmetastatic nasopharyngeal carcinoma. Cancer. 2020;126(10):2163–73.
Ma BBY, Kam MKM, Leung SF, Hui EP, King AD, Chan SL, et al. A phase II study of concurrent cetuximab-cisplatin and intensity-modulated radiotherapy in locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. 2012;23(5):1287–92.
Niu X, Hu C, Kong L. Experience with combination of cetuximab plus intensity-modulated radiotherapy with or without chemotherapy for locoregionally advanced nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2013;139(6):1063–71.
He X, Xu J, Guo W, Jiang X, Wang X, Zong D. Cetuximab in combination with chemoradiation after induction chemotherapy of locoregionally advanced nasopharyngeal carcinoma: preliminary results. Futur Oncol. 2013;9(10):1459–67. https://doi.org/10.2217/fon.13.151.
Lee NY, Zhang Q, Pfister DG, Kim J, Garden AS, Mechalakos J, et al. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): a phase 2 multi-institutional trial. Lancet Oncol. 2012;13(2):172–80.
Sun Y, Hu C, Lin Q, Gao L, Wang J, Zhu X. Nimotuzumab plus chemoradiotherapy versus placebo plus chemoradiotherapy in patients with locally advanced nasopharyngeal carcinoma (NPC): a prospective, randomized-controlled, double-blinded, multicenter phase III clinical trial. J Clin Oncol. 2022;40(26):6001–6001.
Liu Z, Fang F, Chang ET, Adami HO, Ye W. Sibship size, birth order and risk of nasopharyngeal carcinoma and infectious mononucleosis: a nationwide study in Sweden. Int J Epidemiol. 2016;45(3):825–34.
Xue WQ, Wang TM, Huang JW, Zhang JB, He YQ, Wu ZY, et al. A comprehensive analysis of genetic diversity of EBV reveals potential high-risk subtypes associated with nasopharyngeal carcinoma in China. Virus Evol. 2021;7(1):1–10.
Xu M, Yao Y, Chen H, Zhang S, Cao S, Zhang Z, et al. Genome sequencing analysis identifies Epstein–Barr virus subtypes associated with high risk of nasopharyngeal carcinoma. Nat Genet. 2019;51(7):1131–6. https://doi.org/10.1038/s41588-019-0436-5.
Ji MF, Sheng W, Cheng WM, Ng MH, Wu BH, Yu X, et al. Incidence and mortality of nasopharyngeal carcinoma: interim analysis of a cluster randomized controlled screening trial (PRO-NPC-001) in southern China. Ann Oncol. 2019;30(10):1630–7.
Wilson J, Manet E, Gruffat H, Busson P, Blondel M, Fahraeus R. EBNA1: oncogenic activity, immune evasion and biochemical functions provide targets for novel therapeutic strategies against Epstein-Barr virus- associated cancers. Cancers (Basel). 2018;10(4):109. https://doi.org/10.3390/cancers10040109.
Hau PM, Lung HL, Wu M, Tsang CM, Wong KL, Mak NK, et al. Targeting Epstein-Barr virus in nasopharyngeal carcinoma. Front Oncol. 2020;10(May):1–18.
Lo AKF, Dawson CW, Lung HL, Wong KL, Young LS. The role of EBV-encoded LMP1 in the NPC tumor microenvironment: from function to therapy. Front Oncol. 2021;11(February):1–17.
Ke X, Yang YC, Hong SL. EBV-LMP1-targeted DNAzyme restrains nasopharyngeal carcinoma growth in a mouse C666–1 xenograft model. Med Oncol. 2011;28(SUPPL. 1):326–32.
Cao Y, Yang L, Jiang W, Wang X, Liao W, Tan G, et al. Therapeutic evaluation of Epstein-Barr virus-encoded latent membrane protein-1 targeted DNAzyme for treating of nasopharyngeal carcinomas. Mol Ther. 2014;22(2):371–7.
Chia WK, Wang WW, Teo M, Tai WM, Lim WT, Tan EH, et al. A phase II study evaluating the safety and efficacy of an adenovirus-δLMP1-LMP2 transduced dendritic cell vaccine in patients with advanced metastatic nasopharyngeal carcinoma. Ann Oncol. 2012;23(4):997–1005.
Lo EJ, Bell D, Woo JS, Li G, Hanna EY, El-Naggar AK, et al. Human papillomavirus and WHO type I nasopharyngeal carcinoma. Laryngoscope. 2010;120(10):1990–7.
Huang WB, Chan JYW, Liu DL. Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: multicenter study from an endemic area in Southern China. Cancer. 2018;124(3):530–6.
Stenmark MH, McHugh JB, Schipper M, Walline HM, Komarck C, Feng FY, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2014;88(3):580–8. https://doi.org/10.1016/j.ijrobp.2013.11.246.
Robinson M, Suh YE, Paleri V, Devlin D, Ayaz B, Pertl L, et al. Oncogenic human papillomavirus-associated nasopharyngeal carcinoma: an observational study of correlation with ethnicity, histological subtype and outcome in a UK population. Infect Agent Cancer. 2013;8(1):1–7.
Kano M, Kondo S, Wakisaka N, Moriyama-Kita M, Nakanishi Y, Endo K, et al. The influence of human papillomavirus on nasopharyngeal carcinoma in Japan. Auris Nasus Larynx. 2017;44(3):327–32. https://doi.org/10.1016/j.anl.2016.07.015.
Li YY, Chung GTY, Lui VWY, To KF, Ma BBY, Chow C, et al. Exome and genome sequencing of nasopharynx cancer identifies NF-? B pathway activating mutations. Nat Commun. 2017;8:1–10. https://doi.org/10.1038/ncomms14121.
Dai W, Zheng H, Cheung AKL, Tang CSM, Ko JMY, Wong BWY, et al. Whole-exome sequencing identifies MST1R as a genetic susceptibility gene in nasopharyngeal carcinoma. Proc Natl Acad Sci U S A. 2016;113(12):3317–22.
Sasaki MM, Skol AD, Bao R, Rhodes LV, Chambers R, Vokes EE, et al. Integrated genomic analysis suggests MLL3 is a novel candidate susceptibility gene for familial nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2015;24(8):1222–8.
Yu G, Hsu WL, Coghill AE, Yu KJ, Wang CP, Lou PJ, et al. Whole-exome sequencing of nasopharyngeal carcinoma families reveals novel variants potentially involved in nasopharyngeal carcinoma. Sci Rep. 2019;9(1):1–10. https://doi.org/10.1038/s41598-019-46137-4.
Xiao RW, Wang F, Wang TM, Zhang JB, Wu ZY, Deng CM, et al. Rare POLN mutations confer risk for familial nasopharyngeal carcinoma through weakened Epstein-Barr virus lytic replication. eBioMedicine. 2022;84. https://doi.org/10.1016/j.ebiom.2022.104267.
Tsang CM, Lui VWY, Bruce JP, Pugh TJ, Lo KW. Translational genomics of nasopharyngeal cancer. Semin Cancer Biol. 2020;61(August):84–100.
Lee NY, Hum M, Ong PY, Myint MK, Ong EHW, Low KP, et al. Germline variants associated with nasopharyngeal carcinoma predisposition identified through whole-exome sequencing. Cancers (Basel). 2022;14(15). https://doi.org/10.3390/cancers14153680.
Tsao SW, Tsang CM, Lo KW. Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos Trans R Soc B Biol Sci. 2017;372(1732). https://doi.org/10.1098/rstb.2016.0270.
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors reported any conflicts of interest, if any, in their respective ICMJE form. Juan Jose Juarez-Vignon Whaley, Michelle Afkhami, Sagus Sampath, Arya Amini, and Diana Bell have no potential conflicts of interest relevant to this article. Victoria M. Villaflor received the following: research funding—Takeda, Stock Ownership—Johnson and Johnson; Advisory Board—Astra Zeneca.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Juarez-Vignon Whaley, J.J., Afkhami, M., Sampath, S. et al. Early Stage and Locally Advanced Nasopharyngeal Carcinoma Treatment from Present to Future: Where Are We and Where Are We Going?. Curr. Treat. Options in Oncol. 24, 845–866 (2023). https://doi.org/10.1007/s11864-023-01083-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-023-01083-2