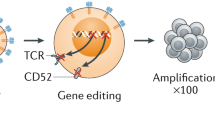
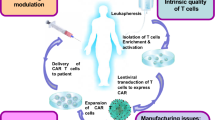
Opinion statement
Chimeric antigen receptor (CAR) cell therapy offers patients with hematological malignancies a new therapeutic option. Traditionally, autologous T cells are used to generate CAR designed T cells for each patient. However, this method has several drawbacks, the development of allogeneic CAR cell therapy would be a promising breakthrough that could address several of these limitations. From the clinical trials that have published data, the efficacy of allogeneic CAR cell therapy did not meet the expectations. Because of the host-versus-graft (HvG) effect, allogeneic CAR cells are eliminated by the host, resulting in short-term persistence of allogeneic CAR cells and poor efficacy. It is critical to solve the HvG effect of allogeneic CAR cells. The current commonly used methods are suppressing the host’s immune system, using HLA-matched homozygous donors, reducing the expression of HLA, targeting alloreactive lymphocytes and eliminating anti-CAR activities. In this review, we will focus on the HvG effect of the “off-the-shelf” allogeneic CAR cell therapy, especially its mechanism and current methods to solve this problem and summarize relevant clinical trial data.



Similar content being viewed by others
Abbreviations
- CAR:
-
Chimeric antigen receptor
- HvG:
-
Host-versus-graft
- CR:
-
Complete remission
- GVHD:
-
Graft-versus-host disease
- allo-HSCT:
-
Allogeneic hematopoietic stem cell transplantation
- B-ALL:
-
B cell acute lymphoblastic leukemia
- DLI:
-
Donor lymphocyte infusion
- UCB:
-
Umbilical cord blood
- iPSC:
-
Induced pluripotent stem cell
- DLT:
-
Dose-limiting toxicity
- CRS:
-
Cytokine release syndrome
- ICANS:
-
Immune effector cell-associated neurotoxicity syndrome\
- PR:
-
Partial remission
- ADCC:
-
Antibody-dependent cell-mediated cytotoxicity
- APC:
-
Antigen-presenting cell
- VST:
-
Virus-specific T cell
- iNKT:
-
Invariant NKT
- MAC:
-
Mesenchymal stem cell
- MMP:
-
Matrix metalloproteinase
- ECM:
-
Extracellular matrix
- PTCL:
-
Peripheral T cell lymphoma
- TRAC:
-
T cell receptor alpha constant
- TRBC:
-
T cell receptor beta constant
- PEBL:
-
Protein expression blocker
- shRNA:
-
Short hairpin RNA
- DSA:
-
Donor-specific anti-HLA antibody
- HAMA:
-
Anti-mouse antibody
- scFv:
-
Single-chain variable fragment
- PNA:
-
Purine nucleotide analogue
- ALCL:
-
Activation-induced C-type lectin
- HCMV:
-
Human cytomegalovirus
- ADR:
-
Alloimmune defense receptor
- CHAR:
-
Chimeric HLA accessory receptor
- RRMM:
-
Recurrent/refractory multiple myeloma
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Zhang X, Lu XA, Yang J, Zhang G, Li J, Song L, et al. Efficacy and safety of anti-CD19 CAR T-cell therapy in 110 patients with B-cell acute lymphoblastic leukemia with high-risk features. Blood Adv. 2020;4(10):2325–38. https://doi.org/10.1182/bloodadvances.2020001466.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. axicabtagene ciloleucel CAR T-cell therapy in refractory large B-Cell lymphoma. N Engl J Med. 2017;377(26):2531–44. https://doi.org/10.1056/NEJMoa1707447.
Zhang M, Jin X, Sun R, Xiong X, Wang J, Xie D, et al. Optimization of metabolism to improve efficacy during CAR-T cell manufacturing. J Transl Med. 2021;19(1):499. https://doi.org/10.1186/s12967-021-03165-x.
Khurana A, Lin Y. Allogeneic Chimeric antigen receptor therapy in lymphoma. Curr Treat Options Oncol. 2022;23(2):171–87. https://doi.org/10.1007/s11864-021-00920-6.
Poehlein CH, Haley DP, Walker EB, Fox BA. Depletion of tumor-induced Treg prior to reconstitution rescues enhanced priming of tumor-specific, therapeutic effector T cells in lymphopenic hosts. Eur J Immunol. 2009;39(11):3121–33. https://doi.org/10.1002/eji.200939453.
Ruella M, Xu J, Barrett DM, Fraietta JA, Reich TJ, Ambrose DE, et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat Med. 2018;24(10):1499–503. https://doi.org/10.1038/s41591-018-0201-9.
•• Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discovery. 2020;19(3):185–99. https://doi.org/10.1038/s41573-019-0051-2. Summarizing the differences between the autologous and allogeneic CAR-T cells, illustrating the potential strategies to improve the GVHD effect and increase the persistence of allogenic CAR-T cells. This review is very comprehensive about allogenic CAR-cell therapy.
Ruella M, Kenderian SS. Next-generation chimeric antigen receptor T-cell therapy: going off the shelf. BioDrugs : Clin Immunotherapeutics, Biopharmaceuticals Gene Ther. 2017;31(6):473–81. https://doi.org/10.1007/s40259-017-0247-0.
Torikai H, Cooper LJ. Translational implications for off-the-shelf immune cells expressing chimeric antigen receptors. Mol Ther: J Am Soc Gene Ther. 2016;24(7):1178–86. https://doi.org/10.1038/mt.2016.106.
• Zhang Y, Li P, Fang H, Wang G, Zeng X. Paving the way towards universal chimeric antigen receptor therapy in cancer treatment: current landscape and progress. Front Immunol. 2020;11:604915. https://doi.org/10.3389/fimmu.2020.604915. Summarizing the methods for reducing GVHD and HvG effects in allogenic CAR-T cells.
June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy: a race to the finish line. Sci Transl Med. 2015;7(280):280ps7. https://doi.org/10.1126/scitranslmed.aaa3643.
Patel K, Bachanova V, Goodman AM, Pagel JM, Griffis K, Anderson M, et al. Phase I Study of FT516, an off-the-shelf iPSC-derived NK cell therapy, in combination with rituximab in patients with relapsed/refractory B-cell lymphoma. Blood. 2021;138(Supplement 1):3873. https://doi.org/10.1182/blood-2021-151520.
Cichocki F, Goodridge JP, Bjordahl R, Gaidarova S, Mahmood S, Abujarour R, et al. Off-the-shelf, multiplexed-engineered iPSC-derived NK cells mediate potent multi-antigen targeting of B-cell malignancies with reduced cytotoxicity against healthy B cells. Blood. 2021;138(Supplement 1):407. https://doi.org/10.1182/blood-2021-148654.
Bachanova V, Ghobadi A, Patel K, Park JH, Flinn IW, Shah P, et al. Safety and efficacy of FT596, a first-in-class, multi-antigen targeted, off-the-shelf, iPSC-derived CD19 CAR NK cell therapy in relapsed/refractory B-cell lymphoma. Blood. 2021;138(Supplement 1):823. https://doi.org/10.1182/blood-2021-151185.
Lin H, Cheng J, Mu W, Zhou J, Zhu L. Advances in universal CAR-T cell therapy. Front Immunol. 2021;12:744823. https://doi.org/10.3389/fimmu.2021.744823.
•• Dhakal B, Chhabra S, Savani BN, Hamadani M. Promise and pitfalls of allogeneic chimeric antigen receptor therapy in plasma cell and lymphoid malignancies. Br J Haematol. 2022;197(1):28–40. https://doi.org/10.1111/bjh.17904. Summerizing some results of partial clinical trials of allogenic CAR-T cell therapy. Explaining the reasons for GVHD and HvG effects of allogenic CAR-T cells and briefly summarizing some solutions.
McCreedy BJ, Senyukov VV, Nguyen KT. Off the shelf T cell therapies for hematologic malignancies. Best Pract Res Clin Haematol. 2018;31(2):166–75. https://doi.org/10.1016/j.beha.2018.03.001.
Zhao J, Lin Q, Song Y, Liu D. Universal CARs, universal T cells, and universal CAR T cells. J Hematol Oncol. 2018;11(1):132. https://doi.org/10.1186/s13045-018-0677-2.
Zhao XY, Xu ZL, Mo XD, Chen YH, Lv M, Cheng YF, et al. Preemptive donor-derived anti-CD19 CAR T-cell infusion showed a promising anti-leukemia effect against relapse in MRD-positive B-ALL after allogeneic hematopoietic stem cell transplantation. Leukemia. 2022;36(1):267–70. https://doi.org/10.1038/s41375-021-01351-w.
Wang B, Iriguchi S, Waseda M, Ueda N, Ueda T, Xu H, et al. Generation of hypoimmunogenic T cells from genetically engineered allogeneic human induced pluripotent stem cells. Nature Biomed Eng. 2021;5(5):429–40. https://doi.org/10.1038/s41551-021-00730-z.
Furukawa Y, Hamano Y, Shirane S, Kinoshita S, Azusawa Y, Ando J, et al. Advances in allogeneic cancer cell therapy and future perspectives on "off-the-shelf" T cell therapy using iPSC technology and gene editing. Cells. 2022;11(2). https://doi.org/10.3390/cells11020269
Li YR, Zhou Y, Kim YJ, Zhu Y, Ma F, Yu J, et al. Development of allogeneic HSC-engineered iNKT cells for off-the-shelf cancer immunotherapy. Cell Rep Med. 2021;2(11):100449. https://doi.org/10.1016/j.xcrm.2021.100449.
Wallet MA, Nishimura T, Del Casale C, Lebid A, Salantes B, Santostefano K, et al. Induced pluripotent stem cell-derived gamma delta CAR-T cells for cancer immunotherapy. Blood. 2021;138(Supplement 1):2771. https://doi.org/10.1182/blood-2021-149095.
Byrne SM, Mali P, Church GM. Genome editing in human stem cells. Methods Enzymol. 2014;546:119–38. https://doi.org/10.1016/b978-0-12-801185-0.00006-4.
van der Stegen SJC, Lindenbergh PL, Petrovic RM, Xie H, Diop MP, Alexeeva V, et al. Generation of T-cell-receptor-negative CD8αβ-positive CAR T cells from T-cell-derived induced pluripotent stem cells. Nat Biomed Eng. 2022. https://doi.org/10.1038/s41551-022-00915-0.
Liu E, Tong Y, Dotti G, Shaim H, Savoldo B, Mukherjee M, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia. 2018;32(2):520–31. https://doi.org/10.1038/leu.2017.226.
Gong Y, Klein Wolterink RGJ, Wang J, Bos GMJ, Germeraad WTV. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J Hematol Oncol. 2021;14(1):73. https://doi.org/10.1186/s13045-021-01083-5.
Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. https://doi.org/10.1146/annurev.immunol.19.1.197.
Guven H, Konstantinidis KV, Alici E, Aints A, Abedi-Valugerdi M, Christensson B, et al. Efficient gene transfer into primary human natural killer cells by retroviral transduction. Exp Hematol. 2005;33(11):1320–8. https://doi.org/10.1016/j.exphem.2005.07.006.
Pan K, Farrukh H, Chittepu V, Xu H, Pan CX, Zhu Z. CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. J Exp Clin Cancer Res: CR. 2022;41(1):119. https://doi.org/10.1186/s13046-022-02327-z.
Deniger DC, Maiti SN, Mi T, Switzer KC, Ramachandran V, Hurton LV, et al. Activating and propagating polyclonal gamma delta T cells with broad specificity for malignancies. Clin Cancer Res: an Off J Am Assoc Cancer Res. 2014;20(22):5708–19. https://doi.org/10.1158/1078-0432.Ccr-13-3451.
Kalyan S, Kabelitz D. Defining the nature of human γδ T cells: a biographical sketch of the highly empathetic. Cell Mol Immunol. 2013;10(1):21–9. https://doi.org/10.1038/cmi.2012.44.
Born WK, Kemal Aydintug M, O’Brien RL. Diversity of γδ T-cell antigens. Cell Mol Immunol. 2013;10(1):13–20. https://doi.org/10.1038/cmi.2012.45.
Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science (New York, NY). 2005;309(5732):264–8. https://doi.org/10.1126/science.1110267.
Nishimoto KP, Barca T, Azameera A, Makkouk A, Romero JM, Bai L, et al. Allogeneic CD20-targeted γδ T cells exhibit innate and adaptive antitumor activities in preclinical B-cell lymphoma models. Clin Transl Immunol. 2022;11(2):e1373. https://doi.org/10.1002/cti2.1373.
Caldwell KJ, Gottschalk S, Talleur AC. Allogeneic CAR cell therapy-more than a pipe dream. Front Immunol. 2020;11:618427. https://doi.org/10.3389/fimmu.2020.618427.
• Mo F, Mamonkin M, Brenner MK, Heslop HE. Taking T-cell oncotherapy off-the-shelf. Trends Immunol. 2021;42(3):261–72. https://doi.org/10.1016/j.it.2021.01.004. Summarizing the methods for reducing GVHD and HvG effects in allogenic CAR-T cells briefly.
Quach DH, Ramos CA, Lulla PD, Sharma S, Ganesh HR, Hadidi YF, et al. Safety and Efficacy of Off-the-Shelf CD30.CAR-Modified Epstein-Barr virus-specific T cells in patients with CD30-positive lymphoma. Blood. 2021;138(Supplement 1):1763. https://doi.org/10.1182/blood-2021-153421.
Quach DH, Ramos CA, Lulla PD, Sharma S, Ganesh HR, Nouraee N, et al. CD30.CAR-modified Epstein-Barr Virus-Specific T Cells (CD30.CAR EBVSTs) provide a safe and effective off-the-shelf therapy for patients with CD30-positive lymphoma. Blood. 2022;140(Supplement 1):412–4. https://doi.org/10.1182/blood-2022-160244.
Exley M, Garcia J, Wilson SB, Spada F, Gerdes D, Tahir SM, et al. CD1d structure and regulation on human thymocytes, peripheral blood T cells, B cells and monocytes. Immunology. 2000;100(1):37–47. https://doi.org/10.1046/j.1365-2567.2000.00001.x.
Chaidos A, Patterson S, Szydlo R, Chaudhry MS, Dazzi F, Kanfer E, et al. Graft invariant natural killer T-cell dose predicts risk of acute graft-versus-host disease in allogeneic hematopoietic stem cell transplantation. Blood. 2012;119(21):5030–6. https://doi.org/10.1182/blood-2011-11-389304.
Leveson-Gower DB, Olson JA, Sega EI, Luong RH, Baker J, Zeiser R, et al. Low doses of natural killer T cells provide protection from acute graft-versus-host disease via an IL-4-dependent mechanism. Blood. 2011;117(11):3220–9. https://doi.org/10.1182/blood-2010-08-303008.
Simonetta F, Lohmeyer JK, Hirai T, Maas-Bauer K, Alvarez M, Wenokur AS, et al. Allogeneic CAR invariant natural killer t cells exert potent antitumor effects through host CD8 T-cell cross-priming. Clin Cancer Res: Off J Am Assoc Cancer Res. 2021;27(21):6054–64. https://doi.org/10.1158/1078-0432.Ccr-21-1329.
Ramos CA, Courtney AN, Robinson SN, Dakhova O, Lulla PD, Kamble R, et al. Allogeneic NKT Cells Expressing a CD19-Specific CAR in Patients with Relapsed or Refractory B-Cell Malignancies: An Interim Analysis. Blood. 2021;138(Supplement 1):2819. https://doi.org/10.1182/blood-2021-149712.
Kot M, Baj-Krzyworzeka M, Szatanek R, Musiał-Wysocka A, Suda-Szczurek M, Majka M. The importance of HLA assessment in "off-the-shelf" allogeneic mesenchymal stem cells based-therapies. Int J Mol Sci 2019;20(22). https://doi.org/10.3390/ijms20225680
Golinelli G, Mastrolia I, Aramini B, Masciale V, Pinelli M, Pacchioni L, et al. Arming mesenchymal stromal/stem cells against cancer: has the time come? Front Pharmacol. 2020;11:529921. https://doi.org/10.3389/fphar.2020.529921.
Li D, Wang W, Xie S, Ge M, Wang R, Xu Q, et al. A T-cell independent universal cellular therapy strategy through antigen depletion. Theranostics. 2022;12(3):1148–60. https://doi.org/10.7150/thno.66832.
Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. 2020;38(8):947–53. https://doi.org/10.1038/s41587-020-0462-y.
Zhang L, Tian L, Dai X, Yu H, Wang J, Lei A, et al. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J Hematol Oncol. 2020;13(1):153. https://doi.org/10.1186/s13045-020-00983-2.
Zhang J, Lei A, Tian L, Zhang L, Lu S, Lu H, et al. The Second generation of human iPSC-derived CAR-macrophages for immune cell therapies in liquid and solid tumors. Blood. 2022;140(Supplement 1):9238–9. https://doi.org/10.1182/blood-2022-165323.
Amrolia PJ, Muccioli-Casadei G, Huls H, Adams S, Durett A, Gee A, et al. Adoptive immunotherapy with allodepleted donor T-cells improves immune reconstitution after haploidentical stem cell transplantation. Blood. 2006;108(6):1797–808. https://doi.org/10.1182/blood-2006-02-001909.
Lekakis LJ, Locke FL, Tees M, Neelapu SS, Malik SA, Hamadani M, et al. ALPHA2 study: ALLO-501A allogeneic CAR T in LBCL, updated results continue to show encouraging safety and efficacy with consolidation dosing. Blood. 2021;138(Supplement 1):649. https://doi.org/10.1182/blood-2021-146045.
Jain N, Roboz GJ, Konopleva M, Liu H, Schiller GJ, Jabbour EJ, et al. Preliminary results from the Flu/Cy/Alemtuzumab arm of the phase I BALLI-01 Trial of UCART22, an Anti-CD22 allogeneic CAR-T cell product, in adult patients with relapsed or refractory (R/R) CD22+ B-Cell acute lymphoblastic leukemia (B-ALL). Blood. 2021;138:1746. https://doi.org/10.1182/blood-2021-150779.
Mailankody S, Liedtke M, Sidana S, Matous JV, Chhabra S, Oluwole OO, et al. Universal updated phase 1 data validates the feasibility of allogeneic anti-BCMA ALLO-715 therapy for relapsed/refractory multiple myeloma. Blood. 2021;138(Supplement 1):651. https://doi.org/10.1182/blood-2021-145572.
Yuan X, Clarke R, Lai Y-S, Chang C-W, Yang B-H, Hsia G, et al. Clinical Manufacture of FT819: use of a clonal multiplexed-engineered master induced pluripotent stem cell line to mass produce off-the-shelf CAR T-cell therapy. Blood. 2021;138(Supplement 1):1766. https://doi.org/10.1182/blood-2021-152985.
Al-Homsi AS, Anguille S, Brayer J, Deeren D, Meuleman N, Morgan G, et al. Clinical development of a non-gene-edited allogeneic Bcma-targeting CAR T-cell product in relapsed or refractory multiple myeloma. Blood. 2020;136(Supplement 1):27–8. https://doi.org/10.1182/blood-2020-139516.
Kamiya T, Wong D, Png YT, Campana D. A novel method to generate T-cell receptor-deficient chimeric antigen receptor T cells. Blood Adv. 2018;2(5):517–28. https://doi.org/10.1182/bloodadvances.2017012823.
Wong XFA, Ng J, Zheng S, Ismail R, Qian H, Campana D, et al. Development of an off-the-shelf chimeric antigen receptor (CAR)-T cell therapy for T-cell acute lymphoblastic leukemia (T-ALL) without gene editing. Blood. 2022;140(Supplement 1):2358–9. https://doi.org/10.1182/blood-2022-165822.
Tonn T, Becker S, Esser R, Schwabe D, Seifried E. Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J Hematother Stem Cell Res. 2001;10(4):535–44. https://doi.org/10.1089/15258160152509145.
• Cutmore LC, Marshall JF. Current perspectives on the use of off the shelf CAR-T/NK Cells for the treatment of cancer. Cancers. 2021;13(8). https://doi.org/10.3390/cancers13081926. Describing the mechanisms of killing tumor cells by other effector cells, such as NK, γδ T, iNKT cells.
Lanza R, Russell DW, Nagy A. Engineering universal cells that evade immune detection. Nat Rev Immunol. 2019;19(12):723–33. https://doi.org/10.1038/s41577-019-0200-1.
Liao NS, Bix M, Zijlstra M, Jaenisch R, Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science (New York, NY). 1991;253(5016):199–202. https://doi.org/10.1126/science.1853205.
Morin-Zorman S, Loiseau P, Taupin JL, Caillat-Zucman S. Donor-specific anti-hla antibodies in allogeneic hematopoietic stem cell transplantation. Front Immunol. 2016;7:307. https://doi.org/10.3389/fimmu.2016.00307.
Wagner DL, Fritsche E, Pulsipher MA, Ahmed N, Hamieh M, Hegde M, et al. Immunogenicity of CAR T cells in cancer therapy. Nat Rev Clin Oncol. 2021;18(6):379–93. https://doi.org/10.1038/s41571-021-00476-2.
Klee GG. Human anti-mouse antibodies. Arch Pathol Lab Med. 2000;124(6):921–3. https://doi.org/10.5858/2000-124-0921-hama.
Maus MV, Haas AR, Beatty GL, Albelda SM, Levine BL, Liu X, et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res. 2013;1:26–31.
Lamers CH, Willemsen R, van Elzakker P, van Steenbergen-Langeveld S, Broertjes M, Oosterwijk-Wakka J, et al. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood. 2011;117(1):72–82. https://doi.org/10.1182/blood-2010-07-294520.
Shah BD, Jacobson C, Solomon SR, Jain N, Johnson MC, Vainorius M, et al. Allogeneic CAR-T PBCAR0191 with intensified lymphodepletion is highly active in patients with relapsed/refractory B-cell malignancies. Blood. 2021;138(Supplement 1):302. https://doi.org/10.1182/blood-2021-150609.
Nie Y, Lu W, Chen D, Tu H, Guo Z, Zhou X, et al. Mechanisms underlying CD19-positive ALL relapse after anti-CD19 CAR T cell therapy and associated strategies. Biomark Res. 2020;8:18. https://doi.org/10.1186/s40364-020-00197-1.
DeSandro A, Nagarajan UM, Boss JM. The bare lymphocyte syndrome: molecular clues to the transcriptional regulation of major histocompatibility complex class II genes. Am J Hum Genet. 1999;65(2):279–86. https://doi.org/10.1086/302519.
Quach DH, Becerra-Dominguez L, Rouce RH, Rooney CM. A strategy to protect off-the-shelf cell therapy products using virus-specific T-cells engineered to eliminate alloreactive T-cells. J Transl Med. 2019;17(1):240. https://doi.org/10.1186/s12967-019-1988-y.
Cheng Y, Zhang J, Wang X, Dong W, Yao X, Zhang Y, et al. Allogeneic CAR-T therapy enabled by base editing of lck to resist dasatinib used to prevent rejection mediated through both T and NK in host. Blood. 2022;140(Supplement 1):4556–7. https://doi.org/10.1182/blood-2022-169039.
Magnani CF, Gaipa G, Lussana F, Belotti D, Gritti G, Napolitano S, et al. Sleeping Beauty-engineered CAR T cells achieve antileukemic activity without severe toxicities. J Clin Investig. 2020;130(11):6021–33. https://doi.org/10.1172/jci138473.
Brudno JN, Somerville RP, Shi V, Rose JJ, Halverson DC, Fowler DH, et al. Allogeneic T cells that express an anti-CD19 Chimeric antigen receptor induce remissions of B-Cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol: Off J Am Soc Clin Oncol. 2016;34(10):1112–21. https://doi.org/10.1200/jco.2015.64.5929.
Sheldon S, Poulton K. HLA typing and its influence on organ transplantation. Methods Mol Biol (Clifton, NJ). 2006;333:157–74. https://doi.org/10.1385/1-59745-049-9:157.
Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, et al. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8(5):409–12. https://doi.org/10.1038/nmeth.1591.
•• Smirnov S, Petukhov A, Levchuk K, Kulemzin S, Staliarova A, Lepik K, et al. Strategies to circumvent the side-effects of immunotherapy using allogeneic CAR-T Cells and boost its efficacy: results of recent clinical trials. Front Immunol. 2021;12:780145. https://doi.org/10.3389/fimmu.2021.780145. Combining with the relevant clinical experimental data, summarizing the allogenic CAR-cells from multiple effector cells. This is of great clinical significance.
Madison BB, Patil D, Richter M, Li X, Tong M, Cranert S, et al. Cas-CLOVER is a novel high-fidelity nuclease for safe and robust generation of T(SCM)-enriched allogeneic CAR-T cells. Mol Ther Nucleic Acids. 2022;13(29):979–95. https://doi.org/10.1016/j.omtn.2022.06.003.
Hu Y, Zhou Y, Zhang M, Zhao H, Wei G, Ge W, et al. Genetically modified CD7-targeting allogeneic CAR-T cell therapy with enhanced efficacy for relapsed/refractory CD7-positive hematological malignancies: a phase I clinical study. Cell Res. 2022. https://doi.org/10.1038/s41422-022-00721-y.
Su H, Na N, Zhang X, Zhao Y. The biological function and significance of CD74 in immune diseases. Inflamm Res: Off J Eur Histamine Res Soc [et al]. 2017;66(3):209–16. https://doi.org/10.1007/s00011-016-0995-1.
Lee J, Sheen JH, Lim O, Lee Y, Ryu J, Shin D, et al. Abrogation of HLA surface expression using CRISPR/Cas9 genome editing: a step toward universal T cell therapy. Sci Rep. 2020;10(1):17753. https://doi.org/10.1038/s41598-020-74772-9.
• Kagoya Y, Guo T, Yeung B, Saso K, Anczurowski M, Wang CH, et al. Genetic ablation of HLA class I, class II, and the T-cell receptor enables allogeneic T cells to be used for adoptive T-cell therapy. Cancer Immunol Res. 2020;8(7):926–36. https://doi.org/10.1158/2326-6066.Cir-18-0508. Describing how to use the method of reducing HLA expression to reduce the generation of HvG effect in allogenic CAR-T cells. Compared with the double knockout of B2M and TRAC, the triple knockout of B2M, CIITA, and TRAC has a better persistence.
Nice TJ, Deng W, Coscoy L, Raulet DH. Stress-regulated targeting of the NKG2D ligand Mult1 by a membrane-associated RING-CH family E3 ligase. J Immunol (Baltimore, Md : 1950). 2010;185(9):5369–76. https://doi.org/10.4049/jimmunol.1000247.
Wang X, Cabrera FG, Sharp KL, Spencer DM, Foster AE, Bayle JH. Engineering tolerance toward allogeneic CAR-T cells by regulation of MHC surface expression with human herpes virus-8 proteins. Mol Ther: J Am Soc Gene Ther. 2021;29(2):718–33. https://doi.org/10.1016/j.ymthe.2020.10.019.
Karpanasamy T, Wawrzyniecka P, Devereaux S, Kassimatis L, Maciocia NC, Pule M, et al. A Novel protein-based approach to generate allogeneic CAR-T Cells with simultaneous TCR and MHC class 1 downregulation. Blood. 2022;140(Supplement 1):636–7. https://doi.org/10.1182/blood-2022-167980.
Braud VM, Allan DS, O’Callaghan CA, Söderström K, D’Andrea A, Ogg GS, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391(6669):795–9. https://doi.org/10.1038/35869.
Guo Y, Xu B, Wu Z, Bo J, Tong C, Chen D, et al. Mutant B2M-HLA-E and B2M-HLA-G fusion proteins protects universal chimeric antigen receptor-modified T cells from allogeneic NK cell-mediated lysis. Eur J Immunol. 2021;51(10):2513–21. https://doi.org/10.1002/eji.202049107.
Hao M, Yin D, Li Y, Gi Y, Tian J, Guo H, et al. P1452: The preliminary safety and efficacy study of Sc-U02, a non-viral genome targeting, Anti-Cd19 Universal Car-T Product, in Relapsed/Refractory (R/R) Diffuse Large B-Cell Lymphoma Patients: Hemasphere. 2022;6(Suppl ):1334–1335, https://doi.org/10.1097/01.HS9.0000848664.50083.13. eCollection 2022 Jun
Xu H, Wang B, Ono M, Kagita A, Fujii K, Sasakawa N, et al. Targeted disruption of HLA genes via CRISPR-Cas9 generates iPSCs with enhanced immune compatibility. Cell Stem Cell. 2019;24(4):566-78.e7. https://doi.org/10.1016/j.stem.2019.02.005.
Lowdell MW, Lamb L, Hoyle C, Velardi A, Prentice HG. Non-MHC-restricted cytotoxic cells: their roles in the control and treatment of leukaemias. Br J Haematol. 2001;114(1):11–24. https://doi.org/10.1046/j.1365-2141.2001.02906.x.
Deuse T, Hu X, Gravina A, Wang D, Tediashvili G, De C, et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol. 2019;37(3):252–8. https://doi.org/10.1038/s41587-019-0016-3.
Johnson A, Wright H, Hu X, van Hoeven PN, Granger B, Liang O, et al. A dual-antigen targeting, hypoimmune allogeneic CAR T to evade innate and adaptive immune rejection and overcome antigen escape. Blood. 2022;140(Supplement 1):4552–3. https://doi.org/10.1182/blood-2022-168251.
Williams AM, Hayama KL, Pan Y, Groff B, Mbofung RM, Chang A, et al. Alloimmune defense receptor harnesses host immune cell activation to potentiate functional persistence and anti-tumor activity of off-the-shelf, cell-based cancer therapy. Blood. 2022;140(Supplement 1):4547–8. https://doi.org/10.1182/blood-2022-167139.
Mo F, Watanabe N, McKenna MK, Hicks MJ, Srinivasan M, Gomes-Silva D, et al. Engineered off-the-shelf therapeutic T cells resist host immune rejection. Nat Biotechnol. 2021;39(1):56–63. https://doi.org/10.1038/s41587-020-0601-5.
Brudno JN, Lam N, Vanasse D, Shen YW, Rose JJ, Rossi J, et al. Safety and feasibility of anti-CD19 CAR T cells with fully human binding domains in patients with B-cell lymphoma. Nat Med. 2020;26(2):270–80. https://doi.org/10.1038/s41591-019-0737-3.
Schneider D, Xiong Y, Hu P, Wu D, Chen W, Ying T, et al. A Unique human immunoglobulin heavy chain variable domain-only CD33 CAR for the treatment of acute myeloid leukemia. Front Oncol. 2018;8:539. https://doi.org/10.3389/fonc.2018.00539.
Zhou Z, Han Y, Pan H-B, Sang C-J, Shi D-L, Feng C, et al. Tri-Specific CD19xCD20xCD22 VHH CAR-T cells (LCAR-AIO) eradicate antigen-heterogeneous b cell tumors, enhance expansion, and prolong persistence in preclinical in vivo models. Blood. 2021;138:1700. https://doi.org/10.1182/blood-2021-150650.
Lam N, Trinklein ND, Buelow B, Patterson GH, Ojha N, Kochenderfer JN. Anti-BCMA chimeric antigen receptors with fully human heavy-chain-only antigen recognition domains. Nat Commun. 2020;11(1):283. https://doi.org/10.1038/s41467-019-14119-9.
Jonnalagadda M, Mardiros A, Urak R, Wang X, Hoffman LJ, Bernanke A, et al. Chimeric antigen receptors with mutated IgG4 Fc spacer avoid fc receptor binding and improve T cell persistence and antitumor efficacy. Mol Ther: J Am Soc Gene Ther. 2015;23(4):757–68. https://doi.org/10.1038/mt.2014.208.
Peraro L, Bourne CM, Dacek MM, Akalin E, Park JH, Smith EL, et al. Incorporation of bacterial immunoevasins to protect cell therapies from host antibody-mediated immune rejection. Mol Ther: J Am Soc Gene Ther. 2021;29(12):3398–409. https://doi.org/10.1016/j.ymthe.2021.06.022.
Acknowledgements
We thank all colleagues at Tianjin First Central Hospital and First Center Clinic College of Tianjin Medical University for related discussions.
Data Availability
The authors declare that data is available.
Funding
This work was supported by grants from the General Project of the National Natural Science Foundation of China (81970180 to MZ), the Science and Technology Project of Tianjin Municipal Health Committee (TJWJ2022QN030 to MZ), Key projects of Tianjin Applied Basic Research and Multi-Investment Fund (21JCZDJC01240), Science and Technology Project of Tianjin Municipal Health Committee (TJWJ2022XK018 to MZ), and the Key Science and Technology Support Project of Tianjin Science and Technology Bureau (20YFZCSY00800 to MZ), as well as Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-056B).
Ethics Approval and Consent to Participate
Not applicable.
Author Contribution
YXA was a major contributor in writing the manuscript. XJ designed the outline of this manuscript. HKZ, MZ, SM have substantively revised it. MFZ, WYL reviewed and amended the draft. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Consent for Publication
Not applicable.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Leukemia.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
An, Y., Jin, X., Zhang, H. et al. “Off-the-Shelf” Allogeneic CAR Cell Therapy—Neglected HvG Effect. Curr. Treat. Options in Oncol. 24, 409–441 (2023). https://doi.org/10.1007/s11864-023-01061-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-023-01061-8