Opinion statement
Small intestine cancer is rare, accounting for approximately 3% of all gastrointestinal malignancies. The most common histological subtypes include adenocarcinoma, neuroendocrine tumours (NETs) and gastrointestinal stromal tumours (GISTs). In localised disease, surgery remains the mainstay of treatment and the best approach to improve survival. Current treatment for small intestine adenocarcinoma (SIA) is extrapolated from small studies and data from colorectal cancer (CRC). There is limited evidence to guide therapy in the adjuvant setting. However, there are small phase II studies in the advanced setting providing evidence for the role of chemotherapy and immunotherapy. There is also limited evidence assessing the efficacy of targeted therapies. Small intestine NETs are rare, with evidence for somatostatin analogue therapy, particularly in the low to intermediate-grade well-differentiated tumours. Poorly differentiated NETs are generally managed with chemotherapy but have worse outcomes compared with well-differentiated NETs. The management of small intestine GISTs is largely targeting KIT mutations with imatinib. Recent trials have provided evidence for effective therapies in imatinib-resistant tumours and the potential role of immunotherapy. The aim of this article was to review the evidence for the current management and recent advances in the management of small intestine adenocarcinoma, NETs and GISTs.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Aparicio T, Pachev A, Laurent-Puig P, Svrcek M. Epidemiology, risk factors and diagnosis of small bowel adenocarcinoma. Cancers. 2022;14(9):2268. This reference is of importance as it is the most recent extensive overview of this disease including epidemiology, risk factors and management.
Surveillance, Epidemiology, and End Results (SEER). Cancer of the small intestine – cancer stat facts. 2022; Available from: https://seer.cancer.gov/statfacts/html/smint.html. Accessed 22 Jul 2022.
••Casali PG, Blay JY, Abecassis N, Bajpai J, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JV, Boye K. Gastrointestinal stromal tumours: ESMO–EURACAN–GENTURIS clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(1):20–33. This reference is of major importance as it is the most up-to-date guidelines for the management of gastrointestinal stromal tumours.
Khosla D, Dey T, Madan R, Gupta R, Goyal S, Kumar N, Kapoor R. Small bowel adenocarcinoma: an overview. World J Gastrointest Oncol. 2022;14(2):413.
Yu J, Refsum E, Perrin V, Helsingen LM, Wieszczy P, Løberg M, et al.. Inflammatory bowel disease and risk of adenocarcinoma and neuroendocrine tumors in the small bowel. Ann Oncol. 2022.
Platoff RM, Kellish AS, Hakim A, Gaughan JP, Atabek UM, Spitz FR, Hong YK. Simple versus radical resection for duodenal adenocarcinoma: a propensity score matched analysis of national cancer database. Am Surg. 2021;87(2):266–75.
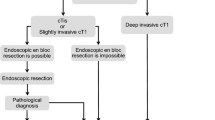
Ochiai Y, Kato M, Kiguchi Y, Akimoto T, Nakayama A, Sasaki M, Fujimoto A, Maehata T, Goto O, Yahagi N. Current status and challenges of endoscopic treatments for duodenal tumors. Digestion. 2019;99(1):21.
Ecker BL, McMillan MT, Datta J, Mamtani R, Giantonio BJ, Dempsey DT, Fraker DL, Drebin JA, Karakousis GC, Roses RE. Efficacy of adjuvant chemotherapy for small bowel adenocarcinoma: a propensity score–matched analysis. Cancer. 2016;122(5):693–701.
Ye X, Zhang G, Chen H, Li Y. Meta-analysis of postoperative adjuvant therapy for small bowel adenocarcinoma. PLoS ONE. 2018;13(8): e0200204.
U.S. National Library of Medicine (NLM) at the National Institute of Health (NIH). Identifier: NCT02502370, Phase III trial investigating the potential benefit of adjuvant chemotherapy for small bowel adenocarcinoma (BALLAD); Clinicaltrials.gov: 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT04257461. Accessed 22 Jul 22.
André T, Meyerhardt J, Iveson T, Sobrero A, Yoshino T, Souglakos I, Grothey A, Niedzwiecki D, Saunders M, Labianca R, Yamanaka T. Effect of duration of adjuvant chemotherapy for patients with stage III colon cancer (IDEA collaboration): final results from a prospective, pooled analysis of six randomised, phase 3 trials. Lancet Oncol. 2020;21(12):1620–9.
Ecker BL, McMillan MT, Datta J, Lee MK, Karakousis GC, Vollmer CM Jr, Drebin JA, Fraker DL, Roses RE. Adjuvant chemotherapy versus chemoradiotherapy in the management of patients with surgically resected duodenal adenocarcinoma: a propensity score-matched analysis of a nationwide clinical oncology database. Cancer. 2017;123(6):967–76.
Onkendi EO, Boostrom SY, Sarr MG, Farnell MB, Nagorney DM, Donohue JH, Kendrick ML, Lombardo KM, Haddock MG, Que FG. Neoadjuvant treatment of duodenal adenocarcinoma: a rescue strategy. J Gastrointest Surg. 2012;16(2):320–4.
Di Nardo P, Garattini SK, Torrisi E, Fanotto V, Miolo G, Buonadonna A, Puglisi F. Systemic treatments for advanced small bowel adenocarcinoma: a systematic review. Cancers. 2022;14(6):1502.
Moati E, Overman MJ, Zaanan A. Therapeutic strategies for patients with advanced small bowel adenocarcinoma: current knowledge and perspectives. Cancers. 2022;14(5):1137.
Xiang XJ, Liu YW, Zhang L, Qiu F, Yu F, Zhan ZY, Feng M, Yan J, Zhao JG, Xiong JP. A phase II study of modified FOLFOX as first-line chemotherapy in advanced small bowel adenocarcinoma. Anticancer Drugs. 2012;23(5):561–6.
Horimatsu T, Nakayama N, Moriwaki T, Hirashima Y, Fujita M, Asayama M, Moriyama I, Nakashima K, Baba E, Kitamura H, Tamura T. A phase II study of 5-fluorouracil/L-leucovorin/oxaliplatin (mFOLFOX6) in Japanese patients with metastatic or unresectable small bowel adenocarcinoma. Int J Clin Oncol. 2017;22(5):905–12.
McWilliams RR, Foster NR, Mahoney MR, Smyrk TC, Murray JA, Ames MM, Horvath LE, Schneider DJ, Hobday TJ, Jatoi A, Meyers JP. North Central Cancer Treatment Group N0543 (Alliance): a phase 2 trial of pharmacogenetic-based dosing of irinotecan, oxaliplatin, and capecitabine as first-line therapy for patients with advanced small bowel adenocarcinoma. Cancer. 2017;123(18):3494–501.
Overman MJ, Varadhachary GR, Kopetz S, Adinin R, Lin E, Morris JS, Eng C, Abbruzzese JL, Wolff RA. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J Clin Oncol. 2009;27(16):2598–603.
Gibson MK, Holcroft CA, Kvols LK, Haller D. Phase II study of 5-fluorouracil, doxorubicin, and mitomycin C for metastatic small bowel adenocarcinoma. Oncologist. 2005;10(2):132–7.
Kim HS, Shin SJ, Kim JH, Kim H, Choi HJ. Better outcome of XELOX chemotherapy in patients with advanced intestinal-type adenocarcinoma of the ampulla of Vater. Tohoku J Exp Med. 2013;231(1):21–8.
Overman MJ, Adam L, Raghav K, Wang J, Kee B, Fogelman D, Eng C, Vilar E, Shroff R, Dasari A, Wolff R. Phase II study of nab-paclitaxel in refractory small bowel adenocarcinoma and CpG island methylator phenotype (CIMP)-high colorectal cancer. Ann Oncol. 2018;29(1):139–44.
Gulhati P, Raghav K, Shroff RT, Varadhachary GR, Kopetz S, Javle M, Qiao W, Wang H, Morris J, Wolff RA, Overman MJ. Bevacizumab combined with capecitabine and oxaliplatin in patients with advanced adenocarcinoma of the small bowel or ampulla of vater: a single-center, open-label, phase 2 study. Cancer. 2017;123(6):1011–7.
Gulhati P, Raghav K, Shroff R, Varadhachary G, Javle M, Qiao W, Wang H, Morris J, Wolff R, Overman MJ. Phase II study of panitumumab in RAS wild-type metastatic adenocarcinoma of small bowel or ampulla of vater. Oncologist. 2018;23(3):277-e26.
Hainsworth JD, Meric-Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C, Burris H, Bose R, Yoo B, Stein A, Beattie M. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an open-label, phase IIa multiple basket study. J Clin Oncol. 2018;36(6):536–44.
••Pedersen KS, Foster NR, Overman MJ, Boland PM, Kim SS, Arrambide KA, Jaszewski BL, Bekaii-Saab T, Graham RP, Welch J, Wilson RH. ZEBRA: a multicenter phase II study of pembrolizumab in patients with advanced small-bowel adenocarcinomapembrolizumab in small-bowel adenocarcinoma. Clinl Cancer Res. 2021;27(13):3641–8. This reference is of importance as it is one of the early studies of immunotherapy in small intestine adenocarcinoma. Several other references below address the same issue.
• Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, Piha-Paul SA. Efficacy of pembrolizumab in patients with non-colorectal high microsatellite instability/mismatch repair–deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38(1):1. This reference is of importance as it highlights the role of immunotherapy in small intestine adenocarcinoma.
Tournigand C, Flechon A, Oudard S, Saada-Bouzid E, Pouessel D, Le Tourneau C, Augereau P, Beylot-Barry M, Grob JJ, Chibaudel B, Soria JC. High level of activity of nivolumab anti-PD-1 immunotherapy and favorable outcome in metastatic/refractory MSI-H non-colorectal cancer: results of the MSI cohort from the French AcSé program. Ann Oncol. 2019;30: v506.
• Chae YK, Othus M, Patel SP, Zalupski M, Kasi A, Khalil M, Kalyan A, Polite B, Fenton S, Gurung S, McLeod CM. Abstract 3417: a phase II basket trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART) SWOG S1609: the small bowel tumor cohort. Cancer Res. 2020;80(16 Suppl):3417. This reference is of importance given the small volume of evidence for immunotherapy in small intestine cancer, as it highlights the role of immunotherapy in small intestine adenocarcinoma.
Cardin DB, Whisenant J, Ayers GD, Gilbert J, Dahlman KB, Shi C, Berlin J. Pilot study to test safety and efficacy of avelumab in small bowel adenocarcinoma (SBA). J Clin Oncol. 2020;38:797.
• Vergara JP, Sacdalan DB, Amurao-Amante M, Sacdalan DL. Bevacizumab in metastatic small-bowel adenocarcinoma: a systematic review and meta-analysis. Rare Tumors. 2019;11:2036361318825413. This reference is of importance as it is a recent meta-analysis that suggests the role of vascular endothelial growth factor inhibitor therapy in small intestine adenocarcinoma.
Zaanan A, Gauthier M, Malka D, Locher C, Gornet JM, Thirot-Bidault A, Tougeron D, Taïeb J, Bonnetain F, Aparicio T, Association des Gastro EntérologuesOncologues. Second-line chemotherapy with fluorouracil, leucovorin, and irinotecan (FOLFIRI regimen) in patients with advanced small bowel adenocarcinoma after failure of first-line platinum-based chemotherapy: a multicenter AGEO study. Cancer. 2011;117(7):1422–8.
U.S. National Library of Medicine (NLM) at the National Institute of Health (NIH). Identifier: NCT04205968. Ramucirumab and Paclitaxel or FOLFIRI in advanced small bowel cancers. Available from: https://clinicaltrials.gov/ct2/show/NCT04205968. Accessed 15 Oct 2022.
Pandya K, Overman MJ, Gulhati P. Molecular landscape of small bowel adenocarcinoma. Cancers. 2022;14(5):1287.
Schrock AB, Devoe CE, McWilliams R, Sun J, Aparicio T, Stephens PJ, Ross JS, Wilson R, Miller VA, Ali SM, Overman MJ. Genomic profiling of small-bowel adenocarcinoma. JAMA Oncol. 2017;3(11):1546–53.
U.S. National Library of Medicine (NLM) at the National Institute of Health (NIH). Identifier: NCT02034110, Efficacy and safety of the combination therapy of Dabrafenib and Trametinib in subjects with BRAF V600E- mutated rare cancers; ClinicalTrials.gov: 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT02034110. Accessed 22 Jul 2022.
• Gelsomino F, Balsano R, De Lorenzo S, Garajová I. Small bowel adenocarcinoma: from molecular insights to clinical management. Curr Oncol. 2022;29(2):1223–36. This reference is of importance as it is a recent study that outlines the spread of genomic changes within small intestine adenocarcinoma.
Holowatyj AN, Washington MK, Tavtigian SV, Eng C, Horton C. Inherited cancer susceptibility gene sequence variations among patients with appendix cancer. JAMA Oncol. 2022.
Lim JY, Pommier RF. Clinical features, management, and molecular characteristics of familial small bowel neuroendocrine tumors. Front Endocrinol. 2021;12: 622693.
O’Toole D, Ducreux M, Bommelaer G, Wemeau JL, Bouché O, Catus F, Blumberg J, Ruszniewski P. Treatment of carcinoid syndrome: a prospective crossover evaluation of lanreotide versus octreotide in terms of efficacy, patient acceptability, and tolerance. Cancer. 2000;88(4):770–6.
Strosberg J, Joish VN, Giacalone S, Perez-Olle R, Fish-Steagall A, Kapoor K, Dharba S, Lapuerta P, Benson AB. TELEPRO: patient-reported carcinoid syndrome symptom improvement following initiation of telotristat ethyl in the real world. Oncologist. 2019;24(11):1446–52.
• Barrett JR, Rendell V, Pokrzywa C, Lopez-Aguiar AG, Cannon J, Poultsides GA, Rocha F, Crown A, Beal E, Michael Pawlik T, Fields R. Adjuvant therapy following resection of gastroenteropancreatic neuroendocrine tumors provides no recurrence or survival benefit. J Surgical Oncol. 2020;121(7):1067–73. This reference is of importance as it provides data in the adjuvant setting for small intestine adenocarcinoma which is an area of unmet need.
Maire F, Hammel P, Kianmanesh R, Hentic O, Couvelard A, Rebours V, Zappa M, Raymond E, Sauvanet A, Louvet C, Lévy P. Is adjuvant therapy with streptozotocin and 5-fluorouracil useful after resection of liver metastases from digestive endocrine tumors? Surgery. 2009;145(1):69–75.
••Pavel M, Oberg K, Falconi M, Krenning EP, Sundin A, Perren A, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. 2020;31(7):844–60. This reference is of major importance as it is the most up-to-date guideline for the management of gastroenteropancreatic neuroendocrine tumours.
Howe JR, Cardona K, Fraker DL, Kebebew E, Untch BR, Wang YZ, et al. The surgical management of small bowel neuroendocrine tumors: consensus guidelines of the North American Neuroendocrine tumor society. Pancreas. 2017;46(6):715–31.
••Zaidi MY, Lopez-Aguiar AG, Dillhoff M, Beal E, Poultsides G, Makris E, et al. Prognostic role of lymph node positivity and number of lymph nodes needed for accurately staging small-bowel neuroendocrine tumors. JAMA Surg. 2019;154(2):134–40. This reference is of major importance as it provides important prognostic information regarding lymph node positivity in small bowel neuroendocrine tumours, which requires standardisation.
Strosberg JR, Coppola D, Klimstra DS, Phan AT, Kulke MH, Wiseman GA, Kvols LK. The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas. 2010;39(6):799.
••Tsilimigras DI, Ntanasis-Stathopoulos I, Kostakis ID, Moris D, Schizas D, Cloyd JM, et al. Is resection of primary midgut neuroendocrine tumors in patients with unresectable metastatic liver disease justified? A Systematic Review and Meta-Analysis. J Gastrointest Surg Of J Soc Surg Aliment Tract. 2019;23(5):1044–54. This reference is of major importance as it addresses the clinically important question regarding the need for resection of the primary tumour.
••Polcz M, Schlegel C, Edwards GC, Wang F, Tan M, Kiernan C, et al. Primary tumor resection offers survival benefit in patients with metastatic midgut neuroendocrine tumors. Ann Surg Oncol. 2020;27(8):2795–803. This reference is of major importance as it addresses the clinically important question regarding the need for resection of the primary tumour.
Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Eng J Med. 2014;371(3):224–33.
Wolin EM, Pavel M, Cwikla JB, Phan AT, Raderer M, Sedlackova E, et al. Abstract 4089: final progression-free survival (PFS) analyses for lanreotide autogel/depot 120 mg in metastatic enteropancreatic neuroendocrine tumors (NETs): The CLARINET extension study. J Clin Oncol. 2017;35 (15 suppl).
Rinke A, Wittenberg M, Schade-Brittinger C, Aminossadati B, Ronicke E, Gress TM, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors (PROMID): results of long-term survival. Neuroendocrinology. 2017;104(1):26–32.
••Strosberg JR, Caplin ME, Kunz PL, Ruszniewski PB, Bodei L, Hendifar A, Mittra E, Wolin EM, Yao JC, Pavel ME, Grande E. 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22(12):1752–63. This reference is of major importance as it is the first study to demonstrate a benefit of a theranostic approach.
Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N Eng J Med. 2017;376(2):125–35.
Pavel ME, Hainsworth JD, Baudin E, Peeters M, Hörsch D, Winkler RE, Klimovsky J, Lebwohl D, Jehl V, Wolin EM, Öberg K. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378(9808):2005–1.
Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, Pacaud LB. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387(10022):968–77.
• Patel SP, Othus M, Chae YK, Giles FJ, Hansel DE, Singh PP, Fontaine A, Shah MH, Kasi A, Baghdadi TA, Matrana M. A phase II basket trial of dual anti–CTLA-4 and anti–PD-1 blockade in rare tumors (DART SWOG 1609) in patients with nonpancreatic neuroendocrine tumors ipilimumab and nivolumab in rare tumors S1609: neuroendocrine. Clin Cancer Res. 2020;26(10):2290–6. This reference is of importance as it is a prominent example of the potential of immunotherapy in neuroendocrine tumours.
Yao JC, Strosberg J, Fazio N, Pavel ME, Bergsland E, Ruszniewski P, Halperin DM, Li D, Tafuto S, Raj N, Campana D. Spartalizumab in metastatic, well/poorly differentiated neuroendocrine neoplasms. Endocr-relat Cancer. 2021;28(3):161–72.
Lu M, Zhang P, Zhang Y, Li Z, Gong J, Li J, Li J, Li Y, Zhang X, Lu Z, Wang X. Efficacy, safety, and biomarkers of toripalimab in patients with recurrent or metastatic neuroendocrine neoplasms: a multiple-center phase Ib trial toripalimab in recurrent or metastatic NENs. Clin Cancer Res. 2020;26(10):2337–45.
Halperin DM, Liu S, Dasari A, Fogelman D, Bhosale P, Mahvash A, et al. Assessment of clinical response following atezolizumab and bevacizumab treatment in patients with neuroendocrine tumors: a nonrandomized clinical trial. JAMA Oncol. 2022.
Capdevila J, Teule A, López C, García-Carbonero R, Benavent M, Custodio A, Cubillo A, Alonso V, Gordoa TA, Carmona-Bayonas A, Crespo G. 1157O A multi-cohort phase II study of durvalumab plus tremelimumab for the treatment of patients (pts) with advanced neuroendocrine neoplasms (NENs) of gastroenteropancreatic or lung origin: The DUNE trial (GETNE 1601). Ann Oncol. 2020;31:S770-1.
U.S. National Library of Medicine (NLM) at the National Institute of Health (NIH). Identifier: NCT04543955. Telotristat with lutathera in neuroendocrine tumors. Available from: https://clinicaltrials.gov/ct2/show/NCT04543955. Accessed 15 Oct 2022.
Delpassand E, Esfandiari R, Tworowska I, Torgue J, Hurt JD, Nunez R. Abstract 4128: Targeted alpha-emitter therapy with 212Pb-DOTAMTATE in neuroendocrine tumor subjects who progressed following prior 177Lu/90Y-PRRT. J Clin Oncol. 2022; 40(16 suppl).
Medley L, Morel AN, Farrugia DF, Reed N, Hayward N, Davies JM, Kirichek O, Thakker RV, Talbot DC. Phase II study of single agent capecitabine in the treatment of metastatic non-pancreatic neuroendocrine tumours. Br J Cancer. 2011;104(7):1067–70.
Jones NB, Shah MH, Bloomston M. Liver-directed therapies in patients with advanced neuroendocrine tumors. J Natl Compr Cancer Netw. 2012;10(6):765–74.
Maxwell JE, Sherman SK, O’Dorisio TM, et al. Liver-directed surgery of neuroendocrine metastases: what is the optimal strategy? Surgery. 2016;159:320–33.
Norlen O, Stålberg P, Zedenius J, Hellman P. Outcome after resection and radiofrequency ablation of liver metastases from small intestinal neuroendocrine tumours. Br J Surg. 2013;100(11):1505–14.
Chan JA, Stuart K, Earle CC, Clark JW, Bhargava P, Miksad R, Blaszkowsky L, Enzinger PC, Meyerhardt JA, Zheng H, Fuchs CS. Prospective study of bevacizumab plus temozolomide in patients with advanced neuroendocrine tumors. J Clin Oncol. 2012;30(24):2963.
Sun W, Lipsitz S, Catalano P, Mailliard JA, Haller DG. Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group Study E1281. J Clin Oncol. 2005;23(22):4897–904.
Ferrarotto R, Testa L, Riechelmann RP, Sahade M, Siqueira LT, Costa FP, Hoff PM. Combination of capecitabine and oxaliplatin is an effective treatment option for advanced neuroendocrine tumors. Rare tumors. 2013;5(3):121–5.
Oziel-Taieb S, Zemmour C, Raoul JL, Mineur L, Poizat F, Charrier N, Piana G, Cavaglione G, Niccoli P. Efficacy of FOLFOX chemotherapy in metastatic enteropancreatic neuroendocrine tumors. Anticancer Res. 2021;41(4):2071–8.
Sahu A, Jefford M, Lai-Kwon J, Thai A, Hicks RJ, Michael M. CAPTEM in metastatic well-differentiated intermediate to high grade neuroendocrine tumors: a single centre experience. J Oncol. 2019;2019.
• Wang W, Zhang Y, Peng Y, Jin KZ, Li YL, Liang Y, Tan HY, Yu XJ, Zhou ZW, Chen J. A Ki-67 index to predict treatment response to the capecitabine/temozolomide regimen in neuroendocrine neoplasms: a retrospective multicenter study. Neuroendocrinology. 2021;111(8):752–63. This reference is of importance as it is the most recent study demonstrating the activity of this regimen.
U.S. National Library of Medicine (NLM) at the National Institute of Health (NIH). Identifier: NCT04919226. Lutetium 177Lu-edotreotide versus best standard of care in well-differentiated aggressive grade-2 and grade-3 gastroenteropancreatic neuroendocrine tumors (GEP-NETs) - COMPOSE (COMPOSE). Available from: https://clinicaltrials.gov/ct2/show/NCT04919226. Accessed 15 Oct 2022.
Morizane C, Machida N, Honma Y, Okusaka T, Boku N, Kato K, et al. Effectiveness of etoposide and cisplatin vs irinotecan and cisplatin therapy for patients with advanced neuroendocrine carcinoma of the digestive system: the TOPIC-NEC phase 3 randomized clinical trial. JAMA Oncol. 2022.
Zhang P, Li J, Li J, Zhang X, Zhou J, Wang X, Peng Z, Shen L, Lu M. Etoposide and cisplatin versus irinotecan and cisplatin as the first-line therapy for patients with advanced, poorly differentiated gastroenteropancreatic neuroendocrine carcinoma: a randomized phase 2 study. Cancer. 2020;1(126):2086–92.
Hadoux J, Malka D, Planchard D, Scoazec JY, Caramella C, Guigay J, Boige V, Leboulleux S, Burtin P, Berdelou A, Loriot Y. Post-first-line FOLFOX chemotherapy for grade 3 neuroendocrine carcinoma. Endocr Relat Cancer. 2015;22(3):289–98.
McGarrah PW, Leventakos K, Hobday TJ, Molina JR, Finnes HD, Westin GF, Halfdanarson TR. Efficacy of second-line chemotherapy in extrapulmonary neuroendocrine carcinoma. Pancreas. 2020;49(4):529.
McNamara MG, Swain J, Craig Z, Sharma R, Faluyi OO, Wadsley J, et al. Abstract 4005: NET-02: a multicenter, randomized, phase II trial of liposomal irinotecan (nal-IRI) and 5-fluorouracil (5-FU)/folinic acid or docetaxel as second-line therapy in patients (pts) with progressive poorly differentiated extra-pulmonary neuroendocrine carcinoma (PD-EP-NEC). J Clin Oncol. 2022; 40(16 suppl).
Albertelli M, Dotto A, Nista F, Veresani A, Patti L, Gay S, Sciallero S, Boschetti M, Ferone D. Present and future of immunotherapy in neuroendocrine tumors. Rev Endocr Metab Disord. 2021;22(3):615–36.
Giuliano K, Nagarajan N, Canner J, Najafian A, Wolfgang C, Schneider E, Meyer C, Lennon AM, Johnston FM, Ahuja N. Gastric and small intestine gastrointestinal stromal tumors: do outcomes differ? J Surg Oncol. 2017;115(3):351.
DeMatteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, Blackstein ME, Blanke CD, von Mehren M, Brennan MF, Patel S. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9669):1097–104.
Joensuu H, Eriksson M, Sundby Hall K, Reichardt A, Hartmann JT, Pink D, Ramadori G, Hohenberger P, Al-Batran SE, Schlemmer M, Bauer S. Adjuvant imatinib for high-risk GI stromal tumor: analysis of a randomized trial. J Clin Oncol. 2016;34(3):244–50.
U.S. National Library of Medicine (NLM) at the National Institute of Health (NIH). Identifier: NCT02413736, Three versus five years of adjuvant imatinib as treatment of patients with operable GIST; ClinicalTrials.gov: 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT02413736. Accessed 22 Jul 2022.
Eisenberg BL, Harris J, Blanke CD, Demetri GD, Heinrich MC, Watson JC, Hoffman JP, Okuno S, Kane JM, Von Mehren M. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 2009;99(1):42–7.
Blesius A, Cassier PA, Bertucci F, Fayette J, Ray-Coquard I, Bui B, Adenis A, Rios M, Cupissol D, Pérol D, Blay JY. Neoadjuvant imatinib in patients with locally advanced non metastatic GIST in the prospective BFR14 trial. BMC Cancer. 2011;11(1):1–7.
Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol. 2010;28(7):1247.
Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, Desai J. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368(9544):1329–38.
Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, Hohenberger P, Leahy M, Von Mehren M, Joensuu H, Badalamenti G. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):295–302.
Reichardt P, Blay JY, Gelderblom H, Schlemmer M, Demetri GD, Bui-Nguyen B, McArthur GA, Yazji S, Hsu Y, Galetic I, Rutkowski P. Phase III study of nilotinib versus best supportive care with or without a TKI in patients with gastrointestinal stromal tumors resistant to or intolerant of imatinib and sunitinib. Ann Oncol. 2012;23(7):1680–7.
Mir O, Cropet C, Toulmonde M, Le Cesne A, Molimard M, Bompas E, Cassier P, Ray-Coquard I, Rios M, Adenis A, Italiano A. Pazopanib plus best supportive care versus best supportive care alone in advanced gastrointestinal stromal tumours resistant to imatinib and sunitinib (PAZOGIST): a randomised, multicentre, open-label phase 2 trial. Lancet Oncol. 2016;17(5):632–41.
••Blay JY, Serrano C, Heinrich MC, Zalcberg J, Bauer S, Gelderblom H, Schöffski P, Jones RL, Attia S, d’Amato G, Chi P. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(7):923–34. This reference is of major importance as it is a randomized control trial that demonstrates the potential of later line therapy in gastrointestinal stromal tumours.
Heinrich MC, Jones RL, von Mehren M, Schöffski P, Serrano C, Kang YK, Cassier PA, Mir O, Eskens F, Tap WD, Rutkowski P. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): a multicentre, open-label, phase 1 trial. Lancet Oncol. 2020;21(7):935–46.
U.S. National Library of Medicine (NLM) at the National Institute of Health (NIH). Identifier: NCT03594422. A Study of HQP1351 in Patients with GIST or Other Solid Tumors. Available from: https://clinicaltrials.gov/ct2/show/NCT03594422. Accessed 15 Oct 2022.
Fairweather M, Balachandran VP, Li GZ, et al. Cytoreductive surgery for metastatic gastrointestinal stromal tumors treated with tyrosine kinase inhibitors: a 2-institutional analysis. Ann Surg. 2018;268:296.
• Singh AS, Hecht JR, Rosen L, Wainberg ZA, Wang X, Douek M, Hagopian A, Andes R, Sauer L, Brackert SR, Chow W. A randomized phase II study of nivolumab monotherapy or nivolumab combined with ipilimumab in patients with advanced gastrointestinal stromal tumors. Clin Cancer Res. 2022;28(1):84–94. This reference is of importance as it raises the possible role of immunotherapy in gastrointestinal stromal tumours.
U.S. National Library of Medicine (NLM) at the National Institute of Health (NIH). Identifier: NCT02406781. Combination of MK3475 and metronomic cyclophosphamide in patients with advanced sarcomas: multicentre phase II trial (PEMBROSARC); ClinicalTrials.gov: 2021. Available from: https://clinicaltrials.gov/ct2/show/NCT02406781. Accessed 30 Aug 2022.
U.S. National Library of Medicine (NLM) at the National Institute of Health (NIH). Identifier: NCT04258956. A study of avelumab in combination with axitinib in patients with unresectable/metastatic gastrointestinal stromal tumor after failure of standard therapy (AXAGIST); ClinicalTrials.gov: 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT04258956. Accessed 30 Aug 2022.
U.S. National Library of Medicine (NLM) at the National Institute of Health (NIH). Identifier: NCT03475953. A phase I/II study of regorafenib plus avelumab in solid tumors (REGOMUNE); ClinicalTrials.gov: 2021. Available from: https://clinicaltrials.gov/ct2/show/NCT03475953. Accessed 30 Aug 2022.
U.S. National Library of Medicine (NLM) at the National Institute of Health (NIH). Identifier: NCT03609424. PDR001 Plus Imatinib for Metastatic or Unresectable GIST; ClinicalTrials.gov: 2021. Available from: https://clinicaltrials.gov/ct2/show/NCT03609424. Accessed 30 Aug 2022.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Rebecca Symons declares that she has no conflict of interest. Daniel Daly declares that he has no conflict of interest. Robert Gandy declares that he has no conflict of interest. David Goldstein declares grants from Bayer, BMS, personal fees from Panbela and personal fees from Boehringer Ingelheim all outside the submitted work. Morteza Aghmesheh declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Lower Gastrointestinal Cancers
Rights and permissions
About this article
Cite this article
Symons, R., Daly, D., Gandy, R. et al. Progress in the Treatment of Small Intestine Cancer. Curr. Treat. Options in Oncol. 24, 241–261 (2023). https://doi.org/10.1007/s11864-023-01058-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-023-01058-3