Opinion statement
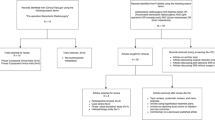
Intracranial stereotactic radiosurgery (SRS) is an effective and convenient treatment for many brain conditions. Data regarding safety come mostly from retrospective single institutional studies and a small number of prospective studies. Variations in target delineation, treatment delivery, imaging follow-up protocols and dose prescription limit the interpretation of this data. There has been much clinical focus on radiation necrosis (RN) in particular, as it is being increasingly recognized on follow-up imaging. Symptomatic RN may be treated with medical therapy (such as corticosteroids and bevacizumab) with surgical resection being reserved for refractory patients. Nevertheless, RN remains a challenging condition to manage, and therefore upfront patient selection for SRS remains critical to provide complication-free control. Mitigation strategies need to be considered in situations where the baseline risk of RN is expected to be high—such as large target volume or re-irradiation. These may involve reduction in the prescribed dose or hypofractionated stereotactic radiation therapy (HSRT). Recently published guidelines and international meta-analysis report the benefit of HSRT in larger lesions, without compromising control rates. However, careful attention to planning parameters and SRS techniques still need to be adhered, even with HSRT. In cases where the risk is deemed to be high despite mitigation, a combination approach of surgery with or without post-operative radiation should be considered.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Barbour AB, Jacobs CD, Williamson H, Floyd SR, Suneja G, Torok JA, et al. Radiation therapy practice patterns for brain metastases in the United States in the stereotactic radiosurgery era. Adv Radiat Oncol. 2020;5(1):43–52. https://doi.org/10.1016/j.adro.2019.07.012.
Kohutek ZA, Yamada Y, Chan TA, Brennan CW, Tabar V, Gutin PH, et al. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neuro-Oncol. 2015;125(1):149–56. https://doi.org/10.1007/s11060-015-1881-3.
Minniti G, Clarke E, Lanzetta G, Osti MF, Trasimeni G, Bozzao A, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;6:48. https://doi.org/10.1186/1748-717X-6-48.
Sneed PK, Mendez J. Vemer-van den Hoek JG, Seymour ZA, Ma L, Molinaro AM et al. Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. J Neurosurg. 2015;123(2):373–86. https://doi.org/10.3171/2014.10.JNS141610.
• Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77(4):996–1001. https://doi.org/10.1016/j.ijrobp.2009.06.006 One of the early studies to suggest irradiated volume to predict for RN.
Schuttrumpf LH, Niyazi M, Nachbichler SB, Manapov F, Jansen N, Siefert A, et al. Prognostic factors for survival and radiation necrosis after stereotactic radiosurgery alone or in combination with whole brain radiation therapy for 1-3 cerebral metastases. Radiat Oncol. 2014;9:105. https://doi.org/10.1186/1748-717X-9-105.
Kim JM, Miller JA, Kotecha R, Xiao R, Juloori A, Ward MC, et al. The risk of radiation necrosis following stereotactic radiosurgery with concurrent systemic therapies. J Neuro-Oncol. 2017;133(2):357–68. https://doi.org/10.1007/s11060-017-2442-8 Large retrospective study from Clevland Clinic looking at the association between RN and the use of various systemic therapeuatic agents.
Calvo W, Hopewell JW, Reinhold HS, Yeung TK. Time- and dose-related changes in the white matter of the rat brain after single doses of X rays. Br J Radiol. 1988;61(731):1043–52. https://doi.org/10.1259/0007-1285-61-731-1043.
Benczik J, Tenhunen M, Snellman M, Joensuu H, Farkkila M, Joensuu R, et al. Late radiation effects in the dog brain: correlation of MRI and histological changes. Radiother Oncol. 2002;63(1):107–20. https://doi.org/10.1016/s0167-8140(02)00028-2.
Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8(2):89–91. https://doi.org/10.1016/j.ccr.2005.07.014.
Kamiryo T, Lopes MB, Kassell NF, Steiner L, Lee KS. Radiosurgery-induced microvascular alterations precede necrosis of the brain neuropil. Neurosurgery. 2001;49(2):409–14; discussion 14-5. https://doi.org/10.1097/00006123-200108000-00026.
Daigle JL, Hong JH, Chiang CS, McBride WH. The role of tumor necrosis factor signaling pathways in the response of murine brain to irradiation. Cancer Res. 2001;61(24):8859–65.
Nordal RA, Nagy A, Pintilie M, Wong CS. Hypoxia and hypoxia-inducible factor-1 target genes in central nervous system radiation injury: a role for vascular endothelial growth factor. Clin Cancer Res. 2004;10(10):3342–53. https://doi.org/10.1158/1078-0432.CCR-03-0426.
Nordal RA, Wong CS. Molecular targets in radiation-induced blood-brain barrier disruption. Int J Radiat Oncol Biol Phys. 2005;62(1):279–87. https://doi.org/10.1016/j.ijrobp.2005.01.039.
Nonoguchi N, Miyatake S, Fukumoto M, Furuse M, Hiramatsu R, Kawabata S, et al. The distribution of vascular endothelial growth factor-producing cells in clinical radiation necrosis of the brain: pathological consideration of their potential roles. J Neuro-Oncol. 2011;105(2):423–31. https://doi.org/10.1007/s11060-011-0610-9.
Burger PC, Mahley MS Jr, Dudka L, Vogel FS. The morphologic effects of radiation administered therapeutically for intracranial gliomas: a postmortem study of 25 cases. Cancer. 1979;44(4):1256–72. https://doi.org/10.1002/1097-0142(197910)44:4<1256::aid-cncr2820440415>3.0.co;2-t.
Panagiotakos G, Alshamy G, Chan B, Abrams R, Greenberg E, Saxena A, et al. Long-term impact of radiation on the stem cell and oligodendrocyte precursors in the brain. PLoS One. 2007;2(7):e588. https://doi.org/10.1371/journal.pone.0000588.
Belka C, Budach W, Kortmann RD, Bamberg M. Radiation induced CNS toxicity--molecular and cellular mechanisms. Br J Cancer. 2001;85(9):1233–9. https://doi.org/10.1054/bjoc.2001.2100.
Hwang SY, Jung JS, Kim TH, Lim SJ, Oh ES, Kim JY, et al. Ionizing radiation induces astrocyte gliosis through microglia activation. Neurobiol Dis. 2006;21(3):457–67. https://doi.org/10.1016/j.nbd.2005.08.006.
Truong MT, St Clair EG, Donahue BR, Rush SC, Miller DC, Formenti SC, et al. Results of surgical resection for progression of brain metastases previously treated by gamma knife radiosurgery. Neurosurgery. 2006;59(1):86–97; discussion 86-97. https://doi.org/10.1227/01.NEU.0000219858.80351.38.
Jagannathan J, Bourne TD, Schlesinger D, Yen CP, Shaffrey ME, Laws ER Jr, et al. Clinical and pathological characteristics of brain metastasis resected after failed radiosurgery. Neurosurgery. 2010;66(1):208–17. https://doi.org/10.1227/01.NEU.0000359318.90478.69.
Barajas RF, Chang JS, Sneed PK, Segal MR, McDermott MW, Cha S. Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol. 2009;30(2):367–72. https://doi.org/10.3174/ajnr.A1362.
Forsyth PA, Kelly PJ, Cascino TL, Scheithauer BW, Shaw EG, Dinapoli RP, et al. Radiation necrosis or glioma recurrence: is computer-assisted stereotactic biopsy useful? J Neurosurg. 1995;82(3):436–44. https://doi.org/10.3171/jns.1995.82.3.0436.
Dequesada IM, Quisling RG, Yachnis A, Friedman WA. Can standard magnetic resonance imaging reliably distinguish recurrent tumor from radiation necrosis after radiosurgery for brain metastases? A radiographic-pathological study. Neurosurgery. 2008;63(5):898–903; discussion 4. https://doi.org/10.1227/01.NEU.0000333263.31870.31.
Chao ST, Ahluwalia MS, Barnett GH, Stevens GH, Murphy ES, Stockham AL, et al. Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int J Radiat Oncol Biol Phys. 2013;87(3):449–57. https://doi.org/10.1016/j.ijrobp.2013.05.015 Concise opinion paper on RN.
Stockham AL, Tievsky AL, Koyfman SA, Reddy CA, Suh JH, Vogelbaum MA, et al. Conventional MRI does not reliably distinguish radiation necrosis from tumor recurrence after stereotactic radiosurgery. J Neuro-Oncol. 2012;109(1):149–58. https://doi.org/10.1007/s11060-012-0881-9.
Zhang Z, Yang J, Ho A, Jiang W, Logan J, Wang X, et al. A predictive model for distinguishing radiation necrosis from tumour progression after gamma knife radiosurgery based on radiomic features from MR images. Eur Radiol. 2018;28(6):2255–63. https://doi.org/10.1007/s00330-017-5154-8.
Zach L, Guez D, Last D, Daniels D, Grober Y, Nissim O, et al. Delayed contrast extravasation MRI: a new paradigm in neuro-oncology. Neuro-Oncology. 2015;17(3):457–65. https://doi.org/10.1093/neuonc/nou230.
Wagner S, Lanfermann H, Eichner G, Gufler H. Radiation injury versus malignancy after stereotactic radiosurgery for brain metastases: impact of time-dependent changes in lesion morphology on MRI. Neuro-Oncology. 2017;19(4):586–94. https://doi.org/10.1093/neuonc/now193.
Alexiou GA, Tsiouris S, Kyritsis AP, Voulgaris S, Argyropoulou MI, Fotopoulos AD. Glioma recurrence versus radiation necrosis: accuracy of current imaging modalities. J Neuro-Oncol. 2009;95(1):1–11. https://doi.org/10.1007/s11060-009-9897-1.
Hu LS, Baxter LC, Smith KA, Feuerstein BG, Karis JP, Eschbacher JM, et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol. 2009;30(3):552–8. https://doi.org/10.3174/ajnr.A1377.
Muto M, Frauenfelder G, Senese R, Zeccolini F, Schena E, Giurazza F, et al. Dynamic susceptibility contrast (DSC) perfusion MRI in differential diagnosis between radionecrosis and neoangiogenesis in cerebral metastases using rCBV, rCBF and K2. Radiol Med. 2018;123(7):545–52. https://doi.org/10.1007/s11547-018-0866-7.
Detsky JS, Keith J, Conklin J, Symons S, Myrehaug S, Sahgal A, et al. Differentiating radiation necrosis from tumor progression in brain metastases treated with stereotactic radiotherapy: utility of intravoxel incoherent motion perfusion MRI and correlation with histopathology. J Neuro-Oncol. 2017;134(2):433–41. https://doi.org/10.1007/s11060-017-2545-2.
Zeng QS, Li CF, Zhang K, Liu H, Kang XS, Zhen JH. Multivoxel 3D proton MR spectroscopy in the distinction of recurrent glioma from radiation injury. J Neuro-Oncol. 2007;84(1):63–9. https://doi.org/10.1007/s11060-007-9341-3.
Shah R, Vattoth S, Jacob R, Manzil FF, O'Malley JP, Borghei P, et al. Radiation necrosis in the brain: imaging features and differentiation from tumor recurrence. Radiographics. 2012;32(5):1343–59. https://doi.org/10.1148/rg.325125002.
Chernov M, Hayashi M, Izawa M, Ochiai T, Usukura M, Abe K, et al. Differentiation of the radiation-induced necrosis and tumor recurrence after gamma knife radiosurgery for brain metastases: importance of multi-voxel proton MRS. Minim Invasive Neurosurg. 2005;48(4):228–34. https://doi.org/10.1055/s-2005-870952.
Mehrabian H, Desmond KL, Soliman H, Sahgal A, Stanisz GJ. Differentiation between radiation necrosis and tumor progression using chemical exchange saturation transfer. Clin Cancer Res. 2017;23(14):3667–75. https://doi.org/10.1158/1078-0432.CCR-16-2265.
Tsuyuguchi N, Sunada I, Iwai Y, Yamanaka K, Tanaka K, Takami T, et al. Methionine positron emission tomography of recurrent metastatic brain tumor and radiation necrosis after stereotactic radiosurgery: is a differential diagnosis possible? J Neurosurg. 2003;98(5):1056–64. https://doi.org/10.3171/jns.2003.98.5.1056.
Tomura N, Kokubun M, Saginoya T, Mizuno Y, Kikuchi Y. Differentiation between treatment-induced necrosis and recurrent tumors in patients with metastatic brain tumors: comparison among (11)C-methionine-PET, FDG-PET, MR permeability imaging, and MRI-ADC-preliminary results. AJNR Am J Neuroradiol. 2017;38(8):1520–7. https://doi.org/10.3174/ajnr.A5252.
Enslow MS, Zollinger LV, Morton KA, Butterfield RI, Kadrmas DJ, Christian PE, et al. Comparison of 18F-fluorodeoxyglucose and 18F-fluorothymidine PET in differentiating radiation necrosis from recurrent glioma. Clin Nucl Med. 2012;37(9):854–61. https://doi.org/10.1097/RLU.0b013e318262c76a.
Heinzel A, Muller D, Yekta-Michael SS, Ceccon G, Langen KJ, Mottaghy FM, et al. O-(2-18F-fluoroethyl)-L-tyrosine PET for evaluation of brain metastasis recurrence after radiotherapy: an effectiveness and cost-effectiveness analysis. Neuro-Oncology. 2017;19(9):1271–8. https://doi.org/10.1093/neuonc/now310.
Li H, Deng L, Bai HX, Sun J, Cao Y, Tao Y, et al. Diagnostic accuracy of amino acid and FDG-PET in differentiating brain metastasis recurrence from radionecrosis after radiotherapy: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2018;39(2):280–8. https://doi.org/10.3174/ajnr.A5472.
Matsunaga S, Shuto T, Takase H, Ohtake M, Tomura N, Tanaka T, et al. Semiquantitative analysis using thallium-201 SPECT for differential diagnosis between tumor recurrence and radiation necrosis after gamma knife surgery for malignant brain tumors. Int J Radiat Oncol Biol Phys. 2013;85(1):47–52. https://doi.org/10.1016/j.ijrobp.2012.03.008.
Milano MT, Grimm J, Niemierko A, Soltys SG, Moiseenko V, Redmond KJ et al. 2020. Single- and multifraction stereotactic radiosurgery dose/volume tolerances of the brain. Int J Radiat Oncol Biol Phys. doi:10.1016/j.ijrobp.2020.08.013. Important publication from the HyTEC group summarising the available evidence of tolerances
Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47(2):291–8. https://doi.org/10.1016/s0360-3016(99)00507-6.
Shehata MK, Young B, Reid B, Patchell RA, St Clair W, Sims J, et al. Stereotatic radiosurgery of 468 brain metastases < or = 2 cm: implications for SRS dose and whole brain radiation therapy. Int J Radiat Oncol Biol Phys. 2004;59(1):87–93. https://doi.org/10.1016/j.ijrobp.2003.10.009.
Shaw E, Scott C, Souhami L, Dinapoli R, Bahary JP, Kline R, et al. Radiosurgery for the treatment of previously irradiated recurrent primary brain tumors and brain metastases: initial report of radiation therapy oncology group protocol (90-05). Int J Radiat Oncol Biol Phys. 1996;34(3):647–54. https://doi.org/10.1016/0360-3016(95)02106-x.
Korytko T, Radivoyevitch T, Colussi V, Wessels BW, Pillai K, Maciunas RJ, et al. 12 Gy gamma knife radiosurgical volume is a predictor for radiation necrosis in non-AVM intracranial tumors. Int J Radiat Oncol Biol Phys. 2006, 64(2):419–24. https://doi.org/10.1016/j.ijrobp.2005.07.980 Pioneering manuscript suggesting the use of the V12 metric for gamma knife treated patients.
Chin LS, Ma L, DiBiase S. Radiation necrosis following gamma knife surgery: a case-controlled comparison of treatment parameters and long-term clinical follow up. J Neurosurg. 2001;94(6):899–904. https://doi.org/10.3171/jns.2001.94.6.0899.
Faruqi S, Ruschin M, Soliman H, Myrehaug S, Zeng KL, Husain Z et al. Adverse radiation effect after hypofractionated stereotactic radiosurgery in 5 daily fractions for surgical cavities and intact brain metastases. International Journal of Radiation Oncology • Biology • Physics. doi:10.1016/j.ijrobp.2019.12.002. Recent publication from Sunnybrooke reporting the outcome of patients treated with 30Gy in 5 fractions (cavity and intact). This paper also suggests tolerances to adhere to during HSRT.
Inoue HK, Seto K, Nozaki A, Torikai K, Suzuki Y, Saitoh J, et al. Three-fraction CyberKnife radiotherapy for brain metastases in critical areas: referring to the risk evaluating radiation necrosis and the surrounding brain volumes circumscribed with a single dose equivalence of 14 Gy (V14). J Radiat Res. 2013;54(4):727–35. https://doi.org/10.1093/jrr/rrt006.
Inoue HK, Sato H, Seto K, Torikai K, Suzuki Y, Saitoh J, et al. Five-fraction CyberKnife radiotherapy for large brain metastases in critical areas: impact on the surrounding brain volumes circumscribed with a single dose equivalent of 14 Gy (V14) to avoid radiation necrosis. J Radiat Res. 2014;55(2):334–42. https://doi.org/10.1093/jrr/rrt127.
Ernst-Stecken A, Ganslandt O, Lambrecht U, Sauer R, Grabenbauer G. Phase II trial of hypofractionated stereotactic radiotherapy for brain metastases: results and toxicity. Radiother Oncol. 2006;81(1):18–24. https://doi.org/10.1016/j.radonc.2006.08.024.
Peng L, Parekh V, Huang P, Lin DD, Sheikh K, Baker B, et al. Distinguishing true progression from radionecrosis after stereotactic radiation therapy for brain metastases with machine learning and radiomics. Int J Radiat Oncol Biol Phys. 2018;102(4):1236–43. https://doi.org/10.1016/j.ijrobp.2018.05.041.
Tsao MN, Rades D, Wirth A, Lo SS, Danielson BL, Gaspar LE, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210–25. https://doi.org/10.1016/j.prro.2011.12.004.
Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15(4):387–95. https://doi.org/10.1016/S1470-2045(14)70061-0.
Hughes RT, Masters AH, McTyre ER, Farris MK, Chung C, Page BR, et al. Initial SRS for patients with 5 to 15 brain metastases: results of a multi-institutional experience. Int J Radiat Oncol Biol Phys. 2019;104(5):1091–8. https://doi.org/10.1016/j.ijrobp.2019.03.052.
Hatiboglu MA, Akdur K. Evaluating critical brain radiation doses in the treatment of multiple brain lesions with gamma knife radiosurgery. Stereotact Funct Neurosurg. 2017;95(4):268–78. https://doi.org/10.1159/000478272.
Minniti G, Capone L, Nardiello B, El Gawhary R, Raza G, Scaringi C, et al. Neurological outcome and memory performance in patients with 10 or more brain metastases treated with frameless linear accelerator (LINAC)-based stereotactic radiosurgery. J Neuro-Oncol. 2020;148(1):47–55. https://doi.org/10.1007/s11060-020-03442-7.
•• Siddiqui ZA, Squires BS, Johnson MD, Baschnagel AM, Chen PY, Krauss DJ, et al. Predictors of radiation necrosis in long-term survivors after gamma knife stereotactic radiosurgery for brain metastases. Neurooncol Pract. 2020;7(4):400–8. https://doi.org/10.1093/nop/npz067 Recent publication from Beaumont, Michigan suggesting that the risk of RN continues to be present for approximately 4 years post SRS, and the risks with re-SRS can be as high as 33%.
Lehrer EJ, Peterson J, Brown PD, Sheehan JP, Quinones-Hinojosa A, Zaorsky NG, et al. Treatment of brain metastases with stereotactic radiosurgery and immune checkpoint inhibitors: an international meta-analysis of individual patient data. Radiother Oncol. 2019;130:104–12. https://doi.org/10.1016/j.radonc.2018.08.025.
Cohen-Inbar O, Shih HH, Xu Z, Schlesinger D, Sheehan JP. The effect of timing of stereotactic radiosurgery treatment of melanoma brain metastases treated with ipilimumab. J Neurosurg. 2017;127(5):1007–14. https://doi.org/10.3171/2016.9.JNS161585.
Skrepnik T, Sundararajan S, Cui H, Stea B. Improved time to disease progression in the brain in patients with melanoma brain metastases treated with concurrent delivery of radiosurgery and ipilimumab. Oncoimmunology. 2017;6(3):e1283461. https://doi.org/10.1080/2162402X.2017.1283461.
Yusuf MB, Amsbaugh MJ, Burton E, Chesney J, Woo S. Peri-SRS Administration of immune checkpoint therapy for melanoma metastatic to the brain: investigating efficacy and the effects of relative treatment timing on lesion response. World Neurosurg. 2017;100:632–40 e4. https://doi.org/10.1016/j.wneu.2017.01.101.
Fang P, Jiang W, Allen P, Glitza I, Guha N, Hwu P, et al. Radiation necrosis with stereotactic radiosurgery combined with CTLA-4 blockade and PD-1 inhibition for treatment of intracranial disease in metastatic melanoma. J Neuro-Oncol. 2017;133(3):595–602. https://doi.org/10.1007/s11060-017-2470-4.
Colaco RJ, Martin P, Kluger HM, Yu JB, Chiang VL. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J Neurosurg. 2016;125(1):17–23. https://doi.org/10.3171/2015.6.JNS142763.
Kaidar-Person O, Zagar TM, Deal A, Moschos SJ, Ewend MG, Sasaki-Adams D, et al. The incidence of radiation necrosis following stereotactic radiotherapy for melanoma brain metastases: the potential impact of immunotherapy. Anti-Cancer Drugs. 2017;28(6):669–75. https://doi.org/10.1097/CAD.0000000000000497.
Minniti G, Anzellini D, Reverberi C, Cappellini GCA, Marchetti L, Bianciardi F, et al. Stereotactic radiosurgery combined with nivolumab or Ipilimumab for patients with melanoma brain metastases: evaluation of brain control and toxicity. J Immunother Cancer. 2019;7(1):102. https://doi.org/10.1186/s40425-019-0588-y.
Martin AM, Cagney DN, Catalano PJ, Alexander BM, Redig AJ, Schoenfeld JD, et al. Immunotherapy and symptomatic radiation necrosis in patients with brain metastases treated with stereotactic radiation. JAMA Oncol. 2018;4(8):1123–4. https://doi.org/10.1001/jamaoncol.2017.3993.
Anker CJ, Grossmann KF, Atkins MB, Suneja G, Tarhini AA, Kirkwood JM. Avoiding severe toxicity from combined BRAF inhibitor and radiation treatment: consensus guidelines from the Eastern Cooperative Oncology Group (ECOG). Int J Radiat Oncol Biol Phys. 2016;95(2):632–46. https://doi.org/10.1016/j.ijrobp.2016.01.038.
Shen CJ, Kummerlowe MN, Redmond KJ, Rigamonti D, Lim MK, Kleinberg LR. Stereotactic radiosurgery: treatment of brain metastasis without interruption of systemic therapy. Int J Radiat Oncol Biol Phys. 2016;95(2):735–42. https://doi.org/10.1016/j.ijrobp.2016.01.054.
Flickinger JC, Kondziolka D, Lunsford LD, Kassam A, Phuong LK, Liscak R, et al. Development of a model to predict permanent symptomatic postradiosurgery injury for arteriovenous malformation patients. Arteriovenous Malformation Radiosurgery Study Group. Int J Radiat Oncol Biol Phys. 2000;46(5):1143–8. https://doi.org/10.1016/s0360-3016(99)00513-1.
Ohtakara K, Hayashi S, Nakayama N, Ohe N, Yano H, Iwama T, et al. Significance of target location relative to the depth from the brain surface and high-dose irradiated volume in the development of brain radionecrosis after micromultileaf collimator-based stereotactic radiosurgery for brain metastases. J Neuro-Oncol. 2012;108(1):201–9. https://doi.org/10.1007/s11060-012-0834-3.
Jhaveri J, Chowdhary M, Zhang X, Press RH, Switchenko JM, Ferris MJ, et al. Does size matter? Investigating the optimal planning target volume margin for postoperative stereotactic radiosurgery to resected brain metastases. J Neurosurg. 2018;130(3):797–803. https://doi.org/10.3171/2017.9.JNS171735.
Kirkpatrick JP, Wang Z, Sampson JH, McSherry F, Herndon JE 2nd, Allen KJ, et al. Defining the optimal planning target volume in image-guided stereotactic radiosurgery of brain metastases: results of a randomized trial. Int J Radiat Oncol Biol Phys. 2015;91(1):100–8. https://doi.org/10.1016/j.ijrobp.2014.09.004.
Raaphorst GP, Malone S, Alsbeih G, Souhani L, Szumacher E, Girard A. Skin fibroblasts in vitro radiosensitivity can predict for late complications following AVM radiosurgery. Radiother Oncol. 2002;64(2):153–6. https://doi.org/10.1016/s0167-8140(02)00076-2.
Zhang Q, Zheng D, Lei Y, Morgan B, Driewer J, Zhang M, et al. A new variable for SRS plan quality evaluation based on normal tissue sparing: the effect of prescription isodose levels. Br J Radiol. 2014;87(1043):20140362. https://doi.org/10.1259/bjr.20140362.
•• Tanenbaum DG, Buchwald ZS, Jhaveri J, Schreibmann E, Switchenko JM, Prabhu RS, et al. Dosimetric factors related to radiation necrosis after 5-fraction radiosurgery for patients with resected brain metastases. Pract Radiat Oncol. 2020;10(1):36–43. https://doi.org/10.1016/j.prro.2019.09.014 Another recent publication reporting the outcome of HSRT and providing suggestions for dose tolerances.
Liu H, Andrews DW, Evans JJ, Werner-Wasik M, Yu Y. Dicker AP et al. Plan quality and treatment efficiency for radiosurgery to multiple brain metastases: non-coplanar RapidArc vs gamma knife Front Oncol. 2016;6:26. https://doi.org/10.3389/fonc.2016.00026.
Ma L, Nichol A, Hossain S, Wang B, Petti P, Vellani R, et al. Variable dose interplay effects across radiosurgical apparatus in treating multiple brain metastases. Int J Comput Assist Radiol Surg. 2014;9(6):1079–86. https://doi.org/10.1007/s11548-014-1001-4.
Solberg TD, Balter JM, Benedict SH, Fraass BA, Kavanagh B, Miyamoto C, et al. Quality and safety considerations in stereotactic radiosurgery and stereotactic body radiation therapy: executive summary. Pract Radiat Oncol. 2012;2(1):2–9. https://doi.org/10.1016/j.prro.2011.06.014.
Wang YX, King AD, Zhou H, Leung SF, Abrigo J, Chan YL, et al. Evolution of radiation-induced brain injury: MR imaging-based study. Radiology. 2010;254(1):210–8. https://doi.org/10.1148/radiol.09090428.
Kotsarini C, Griffiths PD, Wilkinson ID, Hoggard N. A systematic review of the literature on the effects of dexamethasone on the brain from in vivo human-based studies: implications for physiological brain imaging of patients with intracranial tumors. Neurosurgery. 2010;67(6):1799–815; discussion 815. https://doi.org/10.1227/NEU.0b013e3181fa775b.
Tye K, Engelhard HH, Slavin KV, Nicholas MK, Chmura SJ, Kwok Y, et al. An analysis of radiation necrosis of the central nervous system treated with bevacizumab. J Neuro-Oncol. 2014;117(2):321–7. https://doi.org/10.1007/s11060-014-1391-8.
Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(5):1487–95. https://doi.org/10.1016/j.ijrobp.2009.12.061.
Furuse M, Nonoguchi N, Kuroiwa T, Miyamoto S, Arakawa Y, Shinoda J, et al. A prospective, multicentre, single-arm clinical trial of bevacizumab for patients with surgically untreatable, symptomatic brain radiation necrosis(dagger). Neurooncol Pract. 2016;3(4):272–80. https://doi.org/10.1093/nop/npv064.
Brandes AA, Bartolotti M, Tosoni A, Poggi R, Franceschi E. Practical management of bevacizumab-related toxicities in glioblastoma. Oncologist. 2015;20(2):166–75. https://doi.org/10.1634/theoncologist.2014-0330.
Glantz MJ, Burger PC, Friedman AH, Radtke RA, Massey EW, Schold SC Jr. Treatment of radiation-induced nervous system injury with heparin and warfarin. Neurology. 1994;44(11):2020–7. https://doi.org/10.1212/wnl.44.11.2020.
Williamson R, Kondziolka D, Kanaan H, Lunsford LD, Flickinger JC. Adverse radiation effects after radiosurgery may benefit from oral vitamin E and pentoxifylline therapy: a pilot study. Stereotact Funct Neurosurg. 2008;86(6):359–66. https://doi.org/10.1159/000163557.
Ohguri T, Imada H, Kohshi K, Kakeda S, Ohnari N, Morioka T, et al. Effect of prophylactic hyperbaric oxygen treatment for radiation-induced brain injury after stereotactic radiosurgery of brain metastases. Int J Radiat Oncol Biol Phys. 2007;67(1):248–55. https://doi.org/10.1016/j.ijrobp.2006.08.009.
Kohshi K, Imada H, Nomoto S, Yamaguchi R, Abe H, Yamamoto H. Successful treatment of radiation-induced brain necrosis by hyperbaric oxygen therapy. J Neurol Sci. 2003;209(1-2):115–7. https://doi.org/10.1016/s0022-510x(03)00007-8.
Leber KA, Eder HG, Kovac H, Anegg U, Pendl G. Treatment of cerebral radionecrosis by hyperbaric oxygen therapy. Stereotact Funct Neurosurg. 1998;70(Suppl 1):229–36. https://doi.org/10.1159/000056426.
McPherson CM, Warnick RE. Results of contemporary surgical management of radiation necrosis using frameless stereotaxis and intraoperative magnetic resonance imaging. J Neuro-Oncol. 2004;68(1):41–7. https://doi.org/10.1023/b:neon.0000024744.16031.e9.
Nath SK, Sheridan AD, Rauch PJ, Yu JB, Minja FJ, Vortmeyer AO, et al. Significance of histology in determining management of lesions regrowing after radiosurgery. J Neuro-Oncol. 2014;117(2):303–10. https://doi.org/10.1007/s11060-014-1389-2.
Sharma M, Balasubramanian S, Silva D, Barnett GH, Mohammadi AM. Laser interstitial thermal therapy in the management of brain metastasis and radiation necrosis after radiosurgery: An overview. Expert Rev Neurother. 2016;16(2):223–32. https://doi.org/10.1586/14737175.2016.1135736.
Hong CS, Deng D, Vera A, Chiang VL. Laser-interstitial thermal therapy compared to craniotomy for treatment of radiation necrosis or recurrent tumor in brain metastases failing radiosurgery. J Neuro-Oncol. 2019;142(2):309–17. https://doi.org/10.1007/s11060-019-03097-z.
Chaunzwa TL, Deng D, Leuthardt EC, Tatter SB, Mohammadi AM, Barnett GH, et al. Laser thermal ablation for metastases failing radiosurgery: a multicentered retrospective study. Neurosurgery. 2018;82(1):56–63. https://doi.org/10.1093/neuros/nyx142.
•• Ahluwalia M, Barnett GH, Deng D, Tatter SB, Laxton AW, Mohammadi AM, et al. Laser ablation after stereotactic radiosurgery: a multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J Neurosurg. 2018;130(3):804–11. https://doi.org/10.3171/2017.11.JNS171273 Important prospective study investigating the use of LITT to treat both RN and brain tumors.
Minniti G, Scaringi C, Paolini S, Lanzetta G, Romano A, Cicone F, et al. Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys. 2016;95(4):1142–8. https://doi.org/10.1016/j.ijrobp.2016.03.013.
Dore M, Martin S, Delpon G, Clement K, Campion L, Thillays F. Stereotactic radiotherapy following surgery for brain metastasis: predictive factors for local control and radionecrosis. Cancer Radiother. 2017;21(1):4–9. https://doi.org/10.1016/j.canrad.2016.06.010.
•• Lehrer EJ, Peterson JL, Zaorsky NG, Brown PD, Sahgal A, Chiang VL, et al. Single versus multifraction stereotactic radiosurgery for large brain metastases: an international meta-analysis of 24 trials. Int J Radiat Oncol Biol Phys. 2019;103(3):618–30. https://doi.org/10.1016/j.ijrobp.2018.10.038 International MA suggesting that HSRT is safer, and just as effective, for larger lesions.
UK Consortium: Stereotactic ablative body radiation therapy (SABR): a resource. https://www.sabr.org.uk/wp-content/uploads/2019/04/SABRconsortium-guidelines-2019-v6.1.0.pdf. Accessed 12 November 2020
Lawrence YR, Li XA, el Naqa I, Hahn CA, Marks LB, Merchant TE, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S20–7. https://doi.org/10.1016/j.ijrobp.2009.02.091.
Chowdhary M, Okwan-Duodu D, Switchenko JM, Press RH, Jhaveri J, Buchwald ZS, et al. Angiotensin receptor blockade: a novel approach for symptomatic radiation necrosis after stereotactic radiosurgery. J Neuro-Oncol. 2018;136(2):289–98. https://doi.org/10.1007/s11060-017-2652-0.
Montay-Gruel P, Markarian M, Allen BD, Baddour JD, Giedzinski E, Jorge PG, et al. Ultra-high-dose-rate FLASH irradiation limits reactive gliosis in the brain. Radiat Res. 2020. https://doi.org/10.1667/RADE-20-00067.1.
Navarria P, Pessina F, Clerici E, Franceschini D, Gay LG, De Rose F, et al. Surgery followed by hypofractionated radiosurgery on the tumor bed in oligometastatic patients with large brain metastases. Results of a Phase 2 Study. Int J Radiat Oncol Biol Phys. 2019;105(5):1095–105. https://doi.org/10.1016/j.ijrobp.2019.08.054.
Prabhu RS, Patel KR, Press RH, Soltys SG, Brown PD, Mehta MP, et al. Preoperative vs postoperative radiosurgery for resected brain metastases: a review. Neurosurgery. 2019;84(1):19–29. https://doi.org/10.1093/neuros/nyy146.
Ruschin M, Sahgal A, Soliman H, Myrehaug S, Tsao M, Yeboah C, et al. Investigation of irradiated volume in linac-based brain hypo-fractionated stereotactic radiotherapy. Radiat Oncol. 2017;12(1):117. https://doi.org/10.1186/s13014-017-0853-5.
Nedzi LA, Kooy. H, Alexander E, 3rd, Gelman RS, Loeffler JS. Variables associated with the development of complications from radiosurgery of intracranial tumors. Int J Radiat Oncol Biol Phys. 1991;21(3):591–9. https://doi.org/10.1016/0360-3016(91)90675-t.
McDonald D, Schuler J, Takacs I, Peng J, Jenrette J, Vanek K. Comparison of radiation dose spillage from the gamma knife perfexion with that from volumetric modulated arc radiosurgery during treatment of multiple brain metastases in a single fraction. J Neurosurg. 2014;121(Suppl):51–9. https://doi.org/10.3171/2014.7.GKS141358.
Sahgal A, Barani IJ, Novotny J Jr, Zhang B, Petti P, Larson DA, et al. Prescription dose guideline based on physical criterion for multiple metastatic brain tumors treated with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;78(2):605–8. https://doi.org/10.1016/j.ijrobp.2009.11.055.
Tallet AV, Dhermain F, Le Rhun E, Noel G, Kirova YM. Combined irradiation and targeted therapy or immune checkpoint blockade in brain metastases: toxicities and efficacy. Ann Oncol. 2017;28(12):2962–76. https://doi.org/10.1093/annonc/mdx408.
Schoonbeek A, Monshouwer R, Hanssens P, Raaijmakers E, Nowak P, Marijnissen JP, et al. Intracranial radiosurgery in the Netherlands. A planning comparison of available systems with regard to physical aspects and workload. Technol Cancer Res Treat. 2010;9(3):279–90. https://doi.org/10.1177/153303461000900307.
Shaw E, Kline R, Gillin M, Souhami L, Hirschfeld A, Dinapoli R, et al. Radiation therapy oncology group: radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys. 1993;27(5):1231–9. https://doi.org/10.1016/0360-3016(93)90548-a.
Paddick I, Lippitz B. A simple dose gradient measurement tool to complement the conformity index. J Neurosurg. 2006;105(Suppl):194–201. https://doi.org/10.3171/sup.2006.105.7.194.
Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg. 2000;93(Suppl 3):219–22. https://doi.org/10.3171/jns.2000.93.supplement.
Wagner TH, Bova FJ, Friedman WA, Buatti JM, Bouchet LG, Meeks SL. A simple and reliable index for scoring rival stereotactic radiosurgery plans. Int J Radiat Oncol Biol Phys. 2003;57(4):1141–9. https://doi.org/10.1016/s0360-3016(03)01563-3.
Reynolds TA, Jensen AR, Bellairs EE, Ozer M. Dose gradient index for stereotactic radiosurgery/radiation therapy. Int J Radiat Oncol Biol Phys. 2020;106(3):604–11. https://doi.org/10.1016/j.ijrobp.2019.11.408.
Seung SK, Larson DA, Galvin JM, Mehta MP, Potters L, Schultz CJ, et al. American College of Radiology (ACR) and American Society for Radiation Oncology (ASTRO) Practice Guideline for the Performance of Stereotactic Radiosurgery (SRS). Am J Clin Oncol. 2013;36(3):310–5. https://doi.org/10.1097/COC.0b013e31826e053d.
Sahgal A, Kellett S, Ruschin M, Greenspoon J, Follwell M, Sinclair J, et al. A cancer care Ontario organizational guideline for the delivery of stereotactic radiosurgery for brain metastasis in Ontario. Canada Pract Radiat Oncol. 2020;10(4):243–54. https://doi.org/10.1016/j.prro.2019.11.002.
Hartgerink D, Swinnen A, Roberge D, Nichol A, Zygmanski P, Yin FF, et al. LINAC based stereotactic radiosurgery for multiple brain metastases: guidance for clinical implementation. Acta Oncol. 2019;58(9):1275–82. https://doi.org/10.1080/0284186X.2019.1633016.
Growcott S, Dembrey T, Patel R, Eaton D, Cameron A. Inter-observer variability in target volume delineations of benign and metastatic brain tumours for stereotactic radiosurgery: results of a National Quality Assurance Programme. Clin Oncol (R Coll Radiol). 2020;32(1):13–25. https://doi.org/10.1016/j.clon.2019.06.015.
Vellayappan BA, Doody J, Vandervoort E, Szanto J, Sinclair J, Caudrelier JM, et al. Pre-operative versus post-operative radiosurgery for brain metastasis: effects on treatment volume and inter-observer variability. J Radiosurg SBRT. 2018;5(2):89–97.
Rick JW, Shahin M, Chandra A, Dalle Ore C, Yue JK, Nguyen A, et al. Systemic therapy for brain metastases. Crit Rev Oncol Hematol. 2019;142:44–50. https://doi.org/10.1016/j.critrevonc.2019.07.012.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Matthew Foote has received travel and honoraria from Elekta, consulting fee from Varian. Kristin Redmond has received research funding and travel expenses from Accuray, has received research funding from Elekta, has received honorarium from AstraZeneca and acts as a consultant for Medtronic. Simon Lo has received research support from Elekta AB for Gamma Knife ICON Expert Group. Balamurugan A. Vellayappan declares that there is no conflict of interest. Tresa McGranahan declares that there is no conflict of interest. Jerome Graber declares that there is no conflict of interest. Lynne Taylor declares that there is no conflict of interest. Vyshak Venur declares that there is no conflict of interest. Richard Ellenbogen declares that there is no conflict of interest. Andrew E. Sloan declares that there is no conflict of interest. Samuel T. Chao declares that there is no conflict of interest. John H. Suh declares that there is no conflict of interest. Eric L. Chang declares that there is no conflict of interest. Arjun Sahgal declares that there is no conflict of interest.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neuro-oncology
Rights and permissions
About this article
Cite this article
Vellayappan, B.A., McGranahan, T., Graber, J. et al. Radiation Necrosis from Stereotactic Radiosurgery—How Do We Mitigate?. Curr. Treat. Options in Oncol. 22, 57 (2021). https://doi.org/10.1007/s11864-021-00854-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s11864-021-00854-z