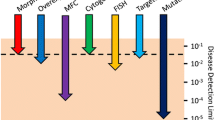
Opinion statement
The expanding availability of minimal or more precisely measurable residual disease (MRD) assessment in acute myeloid leukemia (AML) with its possible implications for therapeutic decisions is of high interest to clinicians treating AML patients. A variety of mostly retrospective studies have shown that AML patients with a positive MRD test, assessed by different techniques at defined cutoffs and time-points, are at significantly higher risk of relapse and experience shorter overall survival compared to MRD-negative patients. How this valuable information may be adapted in the daily routine of patients’ treatment to distinguish individuals who need more aggressive therapy from the ones who can be spared additional therapy to avoid treatment-related toxicities is still being investigated. With the exception of MRD analyses in acute promyelocitic leukemia (APL), the clinical implications of MRD tests for the individual AML patient are still mostly unknown. We currently lack hard evidence that MRD-based therapy modulation during treatment or pre-emptive intervention in MRD-positive patients after therapy would improve outcomes in non-APL AML patients. These questions will be evaluated in prospective randomized clinical trials. Today, however, some conclusions with regard to MRD assessment in AML can be drawn from the published data and are reviewed in this article.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Berry DA, Zhou S, Higley H, Mukundan L, Fu S, Reaman GH, et al. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: a meta-analysis. JAMA Oncol. 2017;3(7):e170580.
Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia. Blood. 2013;122(6):872–84.
Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–52.
Bill M, Grimm J, Jentzsch M, Kloss L, Goldmann K, Schulz J, et al. Digital droplet PCR based absolute quantification of pre-transplant NPM1 mutation burden predicts relapse in acute myeloid leukemia patients. Ann Hematol. 2018;97(10):1757–65.
•• Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–47 The current AML guidelines from the European LeukemiaNet Working Party.
Marcucci G, Mrozek K, Ruppert AS, Archer KJ, Pettenati MJ, Heerema NA, et al. Abnormal cytogenetics at date of morphologic complete remission predicts short overall and disease-free survival, and higher relapse rate in adult acute myeloid leukemia: results from cancer and leukemia group B study 8461. J Clin Oncol. 2004;22(12):2410–8.
Cuneo A, Bigoni R, Roberti MG, Bardi A, Rigolin GM, Piva N, et al. Detection and monitoring of trisomy 8 by fluorescence in situ hybridization in acute myeloid leukemia: a multicentric study. Haematologica. 1998;83(1):21–6.
•• Sanz MA, Fenaux P, Tallman MS, Estey EH, Löwenberg B, Naoe T, et al. Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the European LeukemiaNet. Blood. 2019;133(15):1630–43 The current APL guidelines from the European LeukemiaNet Working Party.
Grimwade D, Jovanovic JV, Hills RK, Nugent EA, Patel Y, Flora R, et al. Prospective minimal residual disease monitoring to predict relapse of acute promyelocytic leukemia and to direct pre-emptive arsenic trioxide therapy. J Clin Oncol. 2009;27(22):3650–8.
•• Schuurhuis GJ, Heuser M, Freeman S, Béné MC, Buccisano F, Cloos J, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2018;131(12):1275–91 The current AML MRD guidelines from the European LeukemiaNet Working Party.
Weisser M, Haferlach C, Hiddemann W, Schnittger S. The quality of molecular response to chemotherapy is predictive for the outcome of AML1-ETO-positive AML and is independent of pretreatment risk factors. Leukemia. 2007;21(6):1177–82.
• Ivey A, Hills RK, Simpson MA, Jovanovic JV, Gilkes A, Grech A, et al. Assessment of minimal residual disease in standard-risk AML. N Engl J Med. 2016;374(5):422–33 A large study showing the applicability of mutated NPM1-MRD in AML patients with intermediate risk.
Balsat M, Renneville A, Thomas X, de Botton S, Caillot D, Marceau A, et al. Postinduction minimal residual disease predicts outcome and benefit from allogeneic stem cell transplantation in acute myeloid leukemia with NPM1 mutation: a study be the Acute Leukemia French Association Group. J Clin Oncol. 2017;35(2):185–93.
Krönke J, Schlenk RF, Jensen KO, Tschürtz F, Corbacioglu A, Gaidzik VI, et al. Monitoring of minimal residual disease in NPM1- mutated acute myeloid leukemia: a study from the German-Austrian acute myeloid leukemia study group. J Clin Oncol. 2011;29(19):2709–16.
Yin JAL, O’Brien MA, Hills RK, Daly SB, Wheatley K, Burnett AK. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: results of the United Kingdom MRC AML-15 trial. Blood. 2012;120(14):2826–35.
Kern W, Schoch C, Haferlach T, Schnittger S. Monitoring of minimal residual disease in acute myeloid leukemia. Crit Rev Oncol Hematol. 2005;56(2):283–309.
Voskova D, Schoch C, Schnittger S, Hiddemann W, Haferlach T, Kern W. Stability of leukemia- associated aberrant immunophenotypes in patients with acute myeloid leukemia between diagnosis and relapse: comparison with cytomorphologic, cytogenetic, and molecular genetic findings. Cytometry B Clin Cytom. 2004;62(1):25–38.
Terwijn M, van Putten WL, Kelder A, van der Velden BH, Brooimans RA, Pabst T, et al. High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: data from the HOVON/SAKK AML 42A study. J Clin Oncol. 2013;31(31):3889–97.
Ravandi F, Jorgensen J, Borthakur G, Jabbour E, Kadia T, Pierce S, et al. Persistence of minimal residual disease assessed by multiparameter flow cytometry is highly prognostic in younger patients with acute myeloid leukemia. Cancer. 2017;123(3):426–35.
Freeman SD, Virgo P, Couzens S, Grimwade D, Russel N, Hills RK, et al. Prognostic relevance of treatment response measured by flow cytometric residual disease detection in older patients with acute myeloid leukemia. J Clin Oncol. 2013;31(32):4123–31.
San Miguel JF, Vidriales MB, López-Berges C, Díaz-Mediavilla J, Gutiérrez N, Cañizo C, et al. Early immunophenotypical evaluation of minimal residual disease in acute myeloid leukemia identifies different patient risk groups and may contribute to postinduction treatment stratification. Blood. 2001;98(6):1746–51.
Walter RB, Buckley SA, Pagel JM, Wood BL, Storer BE, Sandmaier BM, et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood. 2013;122(10):1813–21.
Walter RB, Gooley TA, Wood BL, Milano F, Fang M, Sorror ML, et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol. 2011;29(9):1190–7.
Gabert J, Beillard E, van der Velden VHJ, Bi W, Grimwade D, Pallisgaard N, et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia e a Europe against Cancer program. Leukemia. 2003;17(12):2318e2357.
Takatsuki H, Yufu Y, Tachikawa Y, Uike N. Monitoring minimal residual disease in patients with MLL-AF6 fusion transcript-positive acute myeloid leukemia following allogeneic bone marrow transplantation. Int J Hematol. 2002;75(3):298–301.
Cilloni D, Renneville A, Hermitte F, Hills RK, Daly S, Jovanovic JV, et al. Realtime quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European LeukemiaNet study. J Clin Oncol. 2009;27(31):5195–201.
Lange T, Hubmann M, Burkhardt R, Franke GN, Cross M, Scholz M, et al. Monitoring of WT1 expression in PB and CD34(+) donor chimerism of BM predicts early relapse in AML and MDS patients after hematopoietic cell transplantation with reduced-intensity conditioning. Leukemia. 2011;25(3):498–505.
Weber S, Alpermann T, Dicker F, Jeromin S, Nadarajah N, Eder C, et al. BAALC expression: a suitable marker for prognostic risk stratification and detection of residual disease in cytogenetically normal acute myeloid leukemia. Blood Cancer J. 2014;4:e173.
Jentzsch M, Bill M, Grimm J, Schulz J, Goldmann K, Beinicke S, et al. High blood BAALC copy numbers at allogeneic transplantation predict early relapse in patients with acute myeloid leukemia. Oncotarget. 2017;8(59):87944–54.
Carturan S, Petiti J, Rosso V, Calabrese C, Signorino E, Bot-Sartor G, et al. Variable but consistent pattern of meningioma 1 gene (MN1) expression in different genetic subsets of acute myelogenous leukaemia and its potential use as a marker for minimal residual disease detection. Oncotarget. 2016;7(45):74082–96.
Jentzsch M, Bill M, Grimm J, Schulz J, Beinicke S, Häntschel J, et al. Prognostic impact of blood MN1 copy numbers before allogeneic stem cell transplantation in patients with acute myeloid leukemia. HemaSphere. 2019;3:e167.
Bornhäuser M, Oelschlaegel U, Platzbecker U, Bug G, Lutterbeck K, Kiehl MG, et al. Monitoring of donor chimerism in sorted CD34+ peripheral blood cells allows the sensitive detection of imminent relapse after allogeneic stem cell transplantation. Haematologica. 2009;94(11):1613–7.
Grimwade D, Freeman SD. Defining minimal residual disease in acute myeloid leukemia: which platforms are ready for “prime time”? Blood. 2014;124(23):3345–55.
• Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209–21 This analysis provides an overview of the mutational profile and it’s prognostic significance in AML.
Griffith M, Miller CA, Griffith OL, Krysiak K, Skidmore ZL, Ramu A, et al. Optimizing cancer genome sequencing and analysis. Cell Syst. 2015;1(3):210–23.
• Thol F, Gabdoulline R, Liebich A, Klement P, Schiller J, Kandziora C, et al. Measurable residual disease monitoring by NGS before allogeneic hematopoietic cell transplantation in AML. Blood. 2018;132(16):1703–13. A study showing a high-sensitivity approach utilizing NGS as platform for MRD assessment in AML patients prior to allogeneic transplantation.
• Jongen-Lavrencic M, Grob T, Hanekamp D, Kavelaars FG, Al Hinai A, Zeilemaker A, et al. Molecular minimal residual disease in acute myeloid leukemia. N Engl J Med. 2018;378(13):1189–99 A large multicentric study providing an overview of recurrent mutations in AML suitable for MRD assessment.
Shumilov E, Flach J, Joncourt R, Porret N, Wiedemann G, Angelillo-Scherrer A, et al. Critical evaluation of current molecular MRD strategies including NGS for the management of AML patients with multiple mutations. Hematol Oncol. 2019;37(3):319–22.
• Genovese G, Kähler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477–87 This study describes the frequency and clinical consequence of mutations associated with clonal hematopoiesis in healthy individuals.
Bhatnagar B, Eisfeld AK, Nicolet D, Mrózek K, Blachly JS, Orwick S, et al. Persistence of DNMT3A R882 mutations during remission does not adversely affect outcomes of patients with acute myeloid leukaemia. Br J Haematol. 2016;175(2):226–36.
Pløen GG, Nederby L, Guldberg P, Hansen M, Ebbesen LH, Jensen UB, et al. Persistence of DNMT3A mutations at long-term remission in adult patients with AML. Br J Haematol. 2014;167(4):478–86.
Grimm J, Bill M, Jentzsch M, Beinicke S, Häntschel J, Goldmann K, et al. Clinical impact of clonal hematopoiesis in acute myeloid leukemia patients receiving allogeneic transplantation. Bone Marrow Transplant. 2019;54(8):1189–97.
Rothenberg-Thurley M, Amler S, Goerlich D, Köhnke T, Konstandin NP, Schneider S, et al. Persistence of pre-leukemic clones during first remission and risk of relapse in acute myeloid leukemia. Leukemia. 2018;32(7):1598–608.
Klco JM, Miller CA, Griffith M, Petti A, Spencer DH, Ketkar-Kulkarni S, et al. Association between mutation clearance after induction therapy and outcomes in acute myeloid leukemia. JAMA. 2015;314(8):811–22.
Morita K, Kantarjian HM, Wang F, Yan Y, Bueso-Ramos C, Sasaki K, et al. Clearance of somatic mutations at remission and the risk of relapse in acute myeloid leukemia. J Clin Oncol. 2018;36(18):1788–97.
Willeckens C, Blanchet O, Renneville A, Cornillet-Lefebvre P, Pautas C, Guieze R, et al. Prospective long-term minimal residual disease monitoring using RQ-PCR in RUNX1-RUNX1T1-positive acute myeloid leukemia: results of the French CBF-2006 trial. Haematologica. 2016;101(3):328–35.
Ravandi F, Jorgensen J, Borthakur G, Jabbour E, Kadia T, Pierce S, et al. Persistence of minimal residual disease assessed by multiparameter flow cytometry is highly prognostic in younger patients with acute myeloid leukemia. Cancer. 2017;123(3):426–35.
Buckley SA, Wood BL, Othus M, Hourigan CS, Ustun C, Linden MA, et al. Minimal residual disease prior to allogeneic hematopoietic cell transplantation in acute myeloid leukemia: a meta-analysis. Haematologica. 2017;102(5):865–73.
Kim T, Moon JH, Ahn JS, Kim YK, Lee SS, Ahn SY, et al. Next-generation sequencing-based posttransplant monitoring of acute myeloid leukemia identifies patients at high risk of relapse. Blood. 2018;132(15):1604–13.
Ommen HB, Schnittger S, Jovanovic JV, Ommen IB, Hasle H, Østergaard M, et al. Strikingly different molecular relapse kinetics in NPM1c, PML-RARA, RUNX1-RUNX1T1, and CBFB-MYH11 acute myeloid leukemias. Blood. 2010;115(2):198–205.
Hokland P, Ommen HB. Towards individualized follow-up in adult acute myeloid leukemia in remission. Blood. 2011;117(9):2577–84.
Maurillo L, Buccisano F, Spagnoli A, Del Poeta G, Panetta P, Neri B, et al. Monitoring of minimal residual disease in adult acute myeloid leukemia using peripheral blood as an alternative source to bone marrow. Haematologica. 2007;92(5):605–11.
Zeijlemaker W, Kelder A, Oussoren-Brockhoff YJM, Scholten WJ, Snel AN, Veldhuizen D, et al. Peripheral blood minimal residual disease may replace bone marrow minimal residual disease as an immunophenotypic biomarker for impending relapse in acute myeloid leukemia. Leukemia. 2016;30(3):708–15.
Corbacioglu A, Scholl C, Schlenk RF, Eiwen K, Du J, Bullinger L, et al. Prognostic impact of minimal residual disease in CBFB-MYH11-positive acute myeloid leukemia. J Clin Oncol. 2010;28(23):3724–9.
Boeckx N, De Roover J, van der Velden MJ, Uyttebroeck A, Vandenberghe P, et al. Quantification of CBFB-MYH11 fusion gene levels in paired peripheral blood and bone marrow samples by real-time PCR. Leukemia. 2005;19(11):1988–90.
Ossenkoppele G, Schuurhuis GJ. MRD in AML: does it already guide therapy decision-making? Hematol Am Soc Hematol Educ Program. 2016;2016(1):356–65.
Lo Coco F, Diverio D, Avvisati G, Petti MC, Meloni G, Pogliani EM, et al. Therapy of molecular relapse in acute promyelocytic leukemia. Blood. 1999;94(7):2225–9.
Zhu HH, Zhang XH, Qin YZ, Liu DH, Jiang H, Chen H, et al. MRD-directed risk stratification treatment may improve outcomes of t(8;21) AML in the first complete remission: results from the AML05 multicenter trial. Blood. 2013;121(20):4056–62.
• Platzbecker U, Middeke JM, Sockel K, Herbst R, Wolf D, Baldus CD, et al. Measurable residual disease-guided treatment with azacitidine to prevent haematological relapse in patients with myelodysplastic syndrome and acute myeloid leukaemia (RELAZA2): an open-label, multicentre, phase 2 trial. Lancet Oncol. 2018;19(12):1668–79 A trial showing the feasibility of azacytidine to prevent or delay relapse in AML patients.
Appelbaum FR. Hematopoietic cell transplantation as treatment of patients with acute myeloid leukemia with measurable residual disease after consolidation therapy. Best Pract Res Clin Haematol. 2018;31(4):405–9.
Araki D, Wood BL, Othus M, Radich JP, Halpern AB, Zhou Y, et al. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: time to move toward a minimal residual disease–based definition of complete remission? J Clin Oncol. 2016;34(4):329–36.
Elmaagacli AH, Beelen DW, Kroll M, Trzensky S, Stein C, Schaefer UW. Detection of CBFbeta/MYH11 fusion transcripts in patients with inv(16) acute myeloid leukemia after allogeneic bone marrow or peripheral blood progenitor cell transplantation. Bone Marrow Transplant. 1998;21(2):159–66.
Morschhauser F, Cayuela JM, Martini S, Baruchel A, Rousselot P, Socié G, et al. Evaluation of minimal residual disease using reverse-transcriptase polymerase chain reaction in t(8;21) acute myeloid leukemia; a multicentre study of 51 patients. J Clin Oncol. 2000;18(4):788–94.
Schwind S, Edwards CG, Nicolet D, Mrózek K, Maharry K, Wu YZ, et al. Inv(16)/t(16;16) acute myeloid leukemia with non-type a CBFB-MYH11 fusions associate with distinct clinical and genetic features and lack KIT mutations. Blood. 2013;121(2):385–91.
Jourdan E, Boissel N, Chevret S, Delabesse E, Renneville A, Cornillet P, et al. Prospective evaluation of gene mutations and minimal residual disease in patients with core binding factor acute myeloid leukemia. Blood. 2013;121(12):2213–23.
Lui Yin JA, Frost L. Monitoring AML1-ETO and CBFbeta-MYH11 transcripts in acute myeloid leukemia. Curr Oncol Rep. 2003;5(5):399–404.
Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352(3):254–66.
Grimwade D, Mrózek K. Diagnostic and prognostic value of cytogenetics in acute myeloid leukemia. Hematol Oncol Clin North Am. 2011;25(6):1135–61 vii.
Mencia-Trinchant N, Hu Y, Alas MA, Ali F, Wouters BJ, Lee S, et al. Minimal residual disease monitoring of acute myeloid leukemia by massively multiplex digital PCR in patients with NPM1 mutations. J Mol Diagn. 2017;19(4):537–48.
Hindson CM, Chevillet JR, Briggs HA, Gallichotte EN, Ruf IK, Hindson BJ, et al. Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat Methods. 2013;10(10):1003e1005.
Hubmann M, Köhnke T, Hoster E, Schneider S, Dufour A, Zellmeier E, et al. Molecular response assessment by quantitative real-time polymerase chain reaction after induction therapy in NPM1-mutated patients identifies those at high risk of relapse. Haematologica. 2014;99(8):1317–25.
Chou WC, Tang JL, Wu SJ, Tsay W, Yao M, Huang SY, et al. Clinical implications of minimal residual disease monitoring by quantitative polymerase chain reaction in acute myeloid leukemia patients bearing nucleophosmin (NPM1) mutations. Leukemia. 2007;21(5):998–1004.
Karas M, Steinerova K, Lysak D, Hrabetova M, Jungova A, Sramek J, et al. Pre-transplant quantitative determination of NPM1 mutation significantly predicts outcome of allogeneic hematopoietic stem cell transplantation in patients with normal karyotype AML in complete remission. Anticancer Res. 2016;36(10):5487–98.
Topp MS, Gökbuget N, Zugmaier G, Degenhard E, Goebeler ME, Klinger M, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 2012;120(26):5185–7.
Perl A, Martinelli G, Cortes J, Neubauer A, Berman E, Paolini S, et al. Gilteritinib significantly prolongs overall survival in patients with FLT3-mutated (FLT3mut+) relapsed/refractory acute myeloid leukemia (AML): results from the phase 3 Admital trial. Hemasphere. 2019;3:S1 392–3 [abstract].
• Cortes JE, Khaled S, Martinelli G, Perl AE, Ganguly S, Russell N, et al. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):984–97. The first study to show a significant improvement of survival of a FLT3-inhibitor as monotherapy in relapsed/refractory AML.
Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454–64.
DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable remissions with Ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386–98.
Stein EM, DiNardo CD, Fathi AT, Pollyea DA, Stone RM, Altman JK, et al. Molecular remission and response patterns in patients with mutant-IDH2 acute myeloid leukemia treated with enasidenib. Blood. 2019;133(7):676–87.
Godwin CD, Gale RP, Walter RB. Gemtuzumab ozogamicin in acute myeloid leukemia. Leukemia. 2017;31(9):1855–68.
van de Loosdrecht AA, van Wetering S, Santegoets SJAM, Singh SK, Eeltink CM, den Hartog Y, et al. A novel allogeneic off-the-shelf dendritic cell vaccine for post-remission treatment of elderly patients with acute myeloid leukemia. Cancer Immunol Immunother. 2018;67(10):1505–18.
Freeman SD, Hills RK, Virgo P, Khan N, Couzens S, Dillon R, et al. Measurable residual disease at induction redefines partial response in acute myeloid leukemia and stratifies outcomes in patients at standard risk without NPM1 mutations. J Clin Oncol. 2018;36(15):1486–97.
Acknowledgments
We thank Marius Bill, Juliane Grimm, and Laura Kloss for their hard work on the MRD assays, partly presented also in this manuscript.
Funding
Jose Carreras Leukämie Stiftung 04R/2016; Zusammen gegen den Krebs e.V.
Author information
Authors and Affiliations
Contributions
MJ and SSch wrote the first draft of the manuscript. EB, SSt, CT, and UP contributed to the writing and editing and all authors finalized the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Sebastian Schwind declares that he has no conflict of interest.
Madlen Jentzsch declares that she has no conflict of interest.
Enrica Bach declares that she has no conflict of interest.
Sebastian Stasik declares that he has no conflict of interest.
Christian Thiede is Chief Executive Office of AgenDix GmbH, has received research funding from Novartis and Bayer, and has received compensation from Novartis and Daiichi Sankyo for service as a consultant.
Uwe Platzbecker declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent.
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Leukemia
Rights and permissions
About this article
Cite this article
Schwind, S., Jentzsch, M., Bach, E. et al. Use of Minimal Residual Disease in Acute Myeloid Leukemia Therapy. Curr. Treat. Options in Oncol. 21, 8 (2020). https://doi.org/10.1007/s11864-019-0695-5
Published:
DOI: https://doi.org/10.1007/s11864-019-0695-5