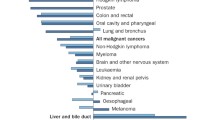
Opinion statement
Hepatocellular carcinoma (HCC) is one of the most lethal cancers globally, particularly in certain regions of the world. Although the major risk factors for HCC have been identified, the specific mechanisms driving hepatocarcinogenesis remain unclear. Sorafenib is the only systemic therapy that has demonstrated an overall survival benefit in patients with advanced HCC and does so primarily through antiangiogenic activity. However, that actual benefit is still relatively small. Extensive research has focused on targeting dysfunctional molecular pathways in HCC. Despite promising preclinical and early-phase studies, other agents have failed to expand upon the efficacy of sorafenib in large-scale randomized trials. As the development of treatment options in the post-sorafenib setting is ongoing, more efforts are being focused on (1) evaluation of molecular agents targeting pathogenic, HCC-specific pathways; (2) the combination of targeted and cytotoxic therapies in selected subgroups; and (3) the combination of systemic and locoregional therapies in various settings. This article provides a review of recently completed and ongoing studies of molecular targeted agents in HCC, including a brief description of the biologic rationale behind these agents.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest have been highlighted as: • Of importance •• Of major importance
International Agency for Research on Cancer. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012 [Internet]. http://globocan.iarc.fr. Available at: http://globocan.iarc.fr/ Accessed March 30, 2014.
Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200.
Liu JH, Chen PW, Asch SM, Busuttil RW, Ko CY. Surgery for hepatocellular carcinoma: does it improve survival? Ann Surg Oncol. 2004;11:298–303.
Mazzaferro V, Llovet JM, Miceli R, Bhoori S. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43.
Ihde DC, Kane RC, Cohen MH, McIntire KR, Minna JD. Adriamycin therapy in American patients with hepatocellular carcinoma. Cancer Treat Rep. 1977;61:1385–7.
Qin S, Bai Y, Lim HY, Thongprasert S, Chao Y, Fan J, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501–8.
Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis. 2010;30:3–16.
El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76.
Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–87. This article provide a review of the etiological factors and associated molecular mechanisms involved in hepatocarcinogenesis.
Shlomai A, de Jong YP, Rice CM. Virus associated malignancies: The role of viral hepatitis in hepatocellular carcinoma. Semin Cancer Biol. 2014. doi:10.1016/j.semcancer.2014.01.004.
Tao Y, Ruan J, Yeh S-H, Lu X, Wang Y, Zhai W, et al. Rapid growth of a hepatocellular carcinoma and the driving mutations revealed by cell-population genetic analysis of whole-genome data. Proc Natl Acad Sci. 2011;108:12042–7.
Zhang DY, Friedman SL. Fibrosis‐dependent mechanisms of hepatocarcinogenesis. Hepatology. 2012;56:769–75.
Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, Maad IB, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44:694–8.
Kan Z, Zheng H, Liu X, Li S, Barbara TD, Gong Z, et al. Whole-genome sequencing identifies recurrent mutations in hepatocellular carcinoma. Genome Res. 2013;23:1422–33.
Aguilar F, Hussain SP, Cerutti P. Aflatoxin B1 induces the transversion of G– > T in codon 249 of the p53 tumor suppressor gene in human hepatocytes. Proc Natl Acad Sci U S A. 1993;90:8586–90.
Tornesello ML, Buonaguro L, Tatangelo F, Botti G, Izzo F, Buonaguro FM. Mutations in TP53, CTNNB1 and PIK3CA genes in hepatocellular carcinoma associated with hepatitis B and hepatitis C virus infections. Genomics. 2013;102:74–83.
Hussain SP, Schwank J, Staib F, Wang XW, Harris CC. TP53 mutations and hepatocellular carcinoma: insights into the etiology and pathogenesis of liver cancer. Oncogene. 2007;26:2166–76.
Matsui O, Kadoya M, Kameyama T, Yoshikawa J, Takashima T, Nakanuma Y, et al. Benign and malignant nodules in cirrhotic livers: distinction based on blood supply. Radiology. 1991;178:493–7.
Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg. 1972;175:409–16.
Zhan P, Qian Q, Yu L-K. Prognostic significance of vascular endothelial growth factor expression in hepatocellular carcinoma tissue: a meta-analysis. Hepatobiliary Surg Nutr. 2013;2:148–55.
Cook KM, Figg WD. Angiogenesis inhibitors: current strategies and future prospects. CA Cancer J Clin. 2010;60:222–43.
Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, et al. Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–55.
Carlomagno F, Anaganti S, Guida T, Salvatore G, Troncone G, Wilhelm SM, et al. BAY 43-9006 inhibition of oncogenic RET mutants. J Natl Cancer Inst. 2006;98:326–34.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. The SHARP trial: this multinational phase III establishes sorafenib as the first systemic therapy to demonstrate an overall survival benefit in advanced HCC patients.
Cheng A-L, Kang Y-K, Chen Z, Tsao C-J, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. The Asia-Pacific trial: this is the correlate phase III study confirming the survival benefit of sorafenib in advanced HCC patients in the Asia-Pacific region. This population was distinct from the SHARP trial, in that it consisted of predominantly HBV-infected patients.
Marrero J, Lencioni R, Ye SL, Kudo M. Final analysis of GIDEON (Global Investigation of Therapeutic Decisions in Hepatocellular Carcinoma [HCC] and of Its Treatment with Sorafenib. J Clin Oncol. 2013;31(Suppl, abstr 4.126). This presentation of the final results from the GIDEON study supports the safety and efficacy of sorafenib in CP-B patients.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–30.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60.
Gillmore R, Stuart S, Kirkwood A, Hameeduddin A, Woodward N, Burroughs AK, et al. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol. 2011;55:1309–16.
Edeline J, Boucher E, Rolland Y, Vauléon E, Pracht M, Perrin C, et al. Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer. 2012;118:147–56.
Llovet JM, Decaens T, Raoul J-L, Boucher E, Kudo M, Chang C, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31:3509–16. This phase III study of brivanib in the second-line setting, following sorafenib failure, failed to achieve significance in overall survival benefit; however, the brivanib arm did show a longer-than-expected survival given the usual natural history of the disease.
Johnson PJ, Qin S, Park J-W, Poon RTP, Raoul J-L, Philip PA, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31:3517–24.
Cheng A-L, Kang Y-K, Lin D-Y, Park J-W, Kudo M, Qin S, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31:4067–75.
Cainap C, Qin S, Huang WT, Chung IJ, Pan H. Phase III trial of linifanib versus sorafenib in patients with advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2013;31(Suppl 4, abstr 249).
Boige V, Malka D, Bourredjem A, Dromain C, Baey C, Jacques N, et al. Efficacy, safety, and biomarkers of single-agent bevacizumab therapy in patients with advanced hepatocellular carcinoma. Oncologist. 2012;17:1063–72.
Zhu AX, Finn RS, Mulcahy M, Gurtler J, Sun W, Schwartz JD, et al. A Phase II and biomarker study of ramucirumab, a human monoclonal antibody targeting the VEGF receptor-2, as first-line monotherapy in patients with advanced hepatocellular cancer. Clin Cancer Res. 2013;19:6614–23.
Goyal L, Muzumdar MD, Zhu AX. Targeting the HGF/c-MET pathway in hepatocellular carcinoma. Clin Cancer Res. 2013;19:2310–8.
Zarnegar R, Michalopoulos GK. The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J Cell. 1995;129:1177–80.
Kaposi-Novak P, Lee J-S, Gòmez-Quiroz L, Coulouarn C, Factor VM, Thorgeirsson SS. Met-regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotype. J Clin Invest. 2006;116:1582–95.
Osada S, Kanematsu M, Imai H, Goshima S. Clinical significance of serum HGF and c-Met expression in tumor tissue for evaluation of properties and treatment of hepatocellular carcinoma. Hepatogastroenterology. 2008;55:544–9.
Ang CS-P, Sun MY, Huitzil-Melendez DF, Chou JF-L, Capanu M, Jarnagin W, et al. c-MET and HGF mRNA expression in hepatocellular carcinoma: correlation with clinicopathological features and survival. Anticancer Res. 2013;33:3241–5.
Santoro A, Rimassa L, Borbath I, Daniele B, Salvagni S, Van Laethem J-L, et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol. 2013;14:55–63. This randomized phase II trial of tivantinib in the second-line setting showed a significant improvement in TTP in a preplanned subset analysis of MET-high patients. It is the basis for an ongoing phase III trial of tivantinib in MET-high proven advanced HCC patients.
Katayama R, Aoyama A, Yamori T, Qi J, Oh-hara T, Song Y, et al. Cytotoxic activity of tivantinib (ARQ 197) is not due solely to c-MET inhibition. Cancer Res. 2013;73:3087–96.
Basilico C, Pennacchietti S, Vigna E, Chiriaco C, Arena S, Bardelli A, et al. Tivantinib (ARQ197) displays cytotoxic activity that is independent of its ability to bind MET. Clin Cancer Res. 2013;19:2381–92.
Cohn AL, Kelley RK, Yang TS, Su WC, Verslype C. Activity of cabozantinib (XL184) in hepatocellular carcinoma patients (pts): results from a phase II randomized discontinuation trial (RDT). J Clin Oncol. 2012;30 (Suppl, abstr 4007).
Schiffer E, Housset C, Cacheux W, Wendum D, Desbois-Mouthon C, Rey C, et al. Gefitinib, an EGFR inhibitor, prevents hepatocellular carcinoma development in the rat liver with cirrhosis. Hepatology. 2005;41:307–14.
O’Dwyer PJ, Giantonio BJ, Levy DE, Kauh JS. Gefitinib in advanced unresectable hepatocellular carcinoma: results from the Eastern Cooperative Oncology Group’s Study E1203. J Clin Oncol. 2006;24(Suppl abstr 4143).
Zhu AX, Rosmorduc O, Evans J, Ross P, Santoro A. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with hepatocellular carcinoma (HCC). Eur J Cancer. 2012; (ESMO, abstr 917).
Govindarajan R, Siegel E, Makhoul I, Williamson S. Bevacizumab and erlotinib in previously untreated inoperable and metastatic hepatocellular carcinoma. Am J Clin Oncol. 2013;36:254–7.
Hsu C-H, Kang Y-K, Yang T-S, Shun C-T, Shao Y-Y, Su W-C, et al. Bevacizumab with erlotinib as first-line therapy in Asian patients with advanced hepatocellular carcinoma: a multicenter phase II study. Oncology. 2013;85:44–52.
Yau T, Wong H, Chan P, Yao TJ, Pang R, Cheung TT, et al. Phase II study of bevacizumab and erlotinib in the treatment of advanced hepatocellular carcinoma patients with sorafenib-refractory disease. Investig New Drugs. 2012;30:2384–90.
Philip PA, Mahoney MR, Holen KD, Northfelt DW, Pitot HC, Picus J, et al. Phase 2 study of bevacizumab plus erlotinib in patients with advanced hepatocellular cancer. Cancer. 2012;118:2424–30.
Kaseb AO, Garrett-Mayer E, Morris JS, Xiao L, Lin E, Onicescu G, et al. Efficacy of bevacizumab plus erlotinib for advanced hepatocellular carcinoma and predictors of outcome: final results of a phase II trial. Oncology. 2012;82:67–74.
Bekaii-Saab T, Markowitz J, Prescott N, Sadee W, Heerema N, Wei L, et al. A multi-institutional phase II study of the efficacy and tolerability of lapatinib in patients with advanced hepatocellular carcinomas. Clin Cancer Res. 2009;15:5895–901.
Zhu AX, Stuart K, Blaszkowsky LS, Muzikansky A, Reitberg DP, Clark JW, et al. Phase 2 study of cetuximab in patients with advanced hepatocellular carcinoma. Cancer. 2007;110:581–9.
Gruenwald V, Wilkens L, Gebel M, Greten TF. A phase II open-label study of cetuximab in unresectable hepatocellular carcinoma: final results. J Clin. 2007;25(18S:4598).
Treiber G. Treatment of advanced or metastatic hepatocellular cancer (HCC): Interim analysis of a single-arm phase II study of bevacizumab and RAD001. J Clin Oncol. 2010;28(Suppl, abstr 255).
Zhu AX, Abrams TA, Miksad R, Blaszkowsky LS, Meyerhardt JA, Zheng H, et al. Phase 1/2 study of everolimus in advanced hepatocellular carcinoma. Cancer. 2011;117:5094–102.
Shiah HS, Chen CY, Dai CY, Hsiao CF, Lin YJ, Su WC, et al. Randomised clinical trial: comparison of two everolimus dosing schedules in patients with advanced hepatocellular carcinoma. Aliment Pharmacol Ther. 2013;37:62–73.
Knox JJ, Qin R, Strosberg JR. A Phase II trial of temsirolimus (TEM) and bevacizumab (BEV) in patients with advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2012;30(Suppl, abstr 4099).
Chelis L, Deftereos S, Xenidis N. Bevacizumab plus temsirolimus as second-line treatment for advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2012;(Suppl, abstr e14567).
Decaens T, Luciani A, Itti E, Hulin A. Phase II study of sirolimus in treatment-naive patients with advanced hepatocellular carcinoma. Digest Liver. 2012;44:610–6.
Chan SL, Yeo W. Targeted therapy of hepatocellular carcinoma: present and future. J Gastroenterol Hepatol. 2012;27:862–72.
Wang S-N, Chuang S-C, Lee K-T. Efficacy of sorafenib as adjuvant therapy to prevent early recurrence of hepatocellular carcinoma after curative surgery: A pilot study. Hepatol Res. 2014;44:523–31. This small pilot study comparing sorafenib following hepatic surgery to surgery alone demonstrated a potential role for sorafenib in the adjuvant setting.
Lencioni R, Llovet JM, Han G, Tak WY, Yang J. Sorafenib or placebo in combination with transarterial chemoembolization (TACE) with doxorubicin-eluting beads (DEBDOX) for intermediate-stage hepatocellular. J Clin. 2012;30(Suppl 4, abstr LBA154). The SPACE trial: this randomized phase II trial showed a minimal but significant improvement in TTP with sorafenib when administered concurrently with TACE with doxorubicin-eluting beads.
Sansonno D, Lauletta G, Russi S, Conteduca V, Sansonno L, Dammacco F. Transarterial chemoembolization plus sorafenib: a sequential therapeutic scheme for HCV-related intermediate-stage hepatocellular carcinoma: a randomized clinical trial. The Oncologist. 2012;17:359–66. This randomized phase II trial demonstrated significant improvement in TTP with sorafenib when administered sequentially following TACE in HCV-related HCC patients.
Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117–27. In this phase III trial in Japan and South Korea, the addition of sorafenib following TACE failed to achieve signifcance when compared to placebo.
Miyahara K, Nouso K, Morimoto Y, Takeuchi Y, Hagihara H, Kuwaki K, et al. Efficacy of sorafenib beyond first progression in patients with metastatic hepatocellular carcinoma. Hepatol Res. 2014;44:296–301.
Rimassa L, Pressiani T, Boni C, Carnaghi C, Rota Caremoli E, Fagiuoli S, et al. A phase II randomized dose escalation trial of sorafenib in patients with advanced hepatocellular carcinoma. Oncologist. 2013;18:379–80.
Abou-Alfa GK, Johnson P, Knox JJ, Capanu M, Davidenko I, Lacava J, et al. Doxorubicin Plus Sorafenib vs Doxorubicin Alone in Patients With Advanced Hepatocellular Carcinoma: A Randomized Trial. JAMA. 2010;304:2154–60.
Hsu CH, Yang TS, Hsu C, Toh HC, Epstein RJ, Hsiao L-T, et al. Efficacy and tolerability of bevacizumab plus capecitabine as first-line therapy in patients with advanced hepatocellular carcinoma. Br J Cancer. 2010;102:981–6.
Sun W, Sohal D, Haller DG, Mykulowycz K, Rosen M, Soulen MC, et al. Phase 2 trial of bevacizumab, capecitabine, and oxaliplatin in treatment of advanced hepatocellular carcinoma. Cancer. 2011;117:3187–92.
Llovet JM, Peña CEA, Lathia CD, Shan M, Meinhardt G, Bruix J, et al. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2012;18:2290–300.
Compliance with Ethics Guidelines
Conflict of Interest
Anuj Patel and Weijing Sun declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, A., Sun, W. Molecular Targeted Therapy in Hepatocellular Carcinoma: From Biology to Clinical Practice and Future. Curr. Treat. Options in Oncol. 15, 380–394 (2014). https://doi.org/10.1007/s11864-014-0291-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-014-0291-7