Abstract
Acute promyelocytic leukaemia (APL) with a STAT5b::RARα gene fusion is an extremely rare subtype of APL characterised by resistance to conventional therapies and extremely poor prognosis. This case highlights that whilst APL with variant RARα translocations are rare, they do pose significant challenges both diagnostically and in their clinical management. This case, in the first instance, demonstrates the importance of using a combination of molecular techniques including next generation sequencing (NGS) for diagnosis particularly in morphological and immunophenotypic typical APL which appears negative by confirmatory testing. Secondly, our patient represents, to the best of our knowledge, the first documented example of this rare disease that has been managed with, and shown sensitivity to low-dose cytarabine (LDAC) in combination with venetoclax (Ven). This case demonstrates that although treatment options are extremely limited for patients not eligible for intensive chemotherapy non-intensive options do show increasing promise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute promyelocytic leukaemia (APL) is a distinct sub-class of acute myeloid leukaemia (AML) as described by the most recent 2022 World Health Organisations (WHO) classification of haematological malignancies [1]. The disease is most commonly cytogenetically characterised by a unique chromosomal translocation involving the retinoic acid receptor alpha (RARα) gene on chromosome 17 and the promyelocytic leukaemia (PML) gene on chromosome 15, resulting in the formation of the PML::RARα gene fusion [2]. This understanding of the pathogenesis of APL has led to the introduction of highly effective targeted therapies such as all-trans retinoic acid (ATRA) and arsenic trioxide (ATO) [3]. The use of these agents has been shown to be remarkably successful when treating non-high-risk APL patients with an event free survival after 72 months of 96.6% having been achieved [4].
The PML::RARα fusion gene is detectable in approximately 98% of patients with APL using fluorescence in situ hybridisation (FISH) or reverse transcription polymerase chain reaction (RT-PCR). However, in the remaining 1–2% of cases of morphologically typical APL novel, extremely rare fusion proteins have been identified involving RARα and cryptic gene partners excluding PML. These are often clustered according to their sensitivity to ATRA therapy. Those that are sensitive include NPM1::RARα and NuMa::RARα and those that are resistant include the fusion genes; PLZF::RARα, ZBTB16::RARα and STAT5b::RARα [5]. The latter are also frequently resistant to ATO and standard induction chemotherapy regimens and are therefore associated with much worse outcomes [6].
The APL variant characterised by STAT5b::RARα fusion gene is an extremely rare form of APL accounting for approximately 0.28% of APL patients [7] with only 18 cases having been reported in the literature [6] (Table 1). Patients with this variant typically present with morphologic and immunophenotypic features comparable to PML::RARα positive APL. This variant is more commonly seen in males and has a poor prognosis [7]. Due to its infrequency and the limited number of documented cases, there is little evidence for the most appropriate treatment.
Case report and methods
A 60-year-old male presented to the local accident and emergency department feeling generally unwell with shortness of breath, icteric sclera, and jaundice. Initial full blood count (FBC) showed pancytopenia, haemoglobin 100 g/L, white cell count 1.3 × 109/L, platelet count 30 × 109/L, and an absolute neutrophil count (ANC) of 0.4 × 109/L. A blood film performed on peripheral blood (PB) revealed a leucoerythroblastic picture and pancytopenia. The coagulation screen and kidney function tests were normal. Serum lactate dehydrogenase (LDH) and C-reactive protein (CRP) were 492 U/L and 28 mg/L respectively. The patient had an extensive past medical history including non-ST segment elevation myocardial infarction (NSTEMI), obesity, type 2 diabetes mellitus (T2DM), hypertension, atrial fibrillation, and recurrent cellulitis. A bone marrow aspirate and trephine was performed which was grossly hypercellular, almost entirely replaced with medium/large blasts/promyelocytes with variable amounts of cytoplasm containing fine azurophilic granules, some with budded cytoplasm and many with a folded nucleus (Fig. 1A and B). These morphologic features raised suspicion of APL. Immunophenotyping analysis was performed and indicated that the blasts/promyelocytes were CD34-, HLA-DR(weak) + , MPO + , CD64 + , CD13 + ,CD33 + , and CD117 + myeloid lineage cells. As a consequence of what appeared to be classic APL morphology and immunophenotyping results, the patient was commenced on ATRA 45 mg/m2, awaiting urgent FISH and real-time quantitative PCR (RQ-PCR).
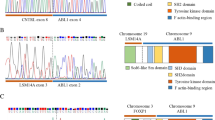
Initial bone marrow aspirate morphology and cytogenetic analysis of the patient. A and B Bone marrow aspirate showing hypercellularity and a population of medium-sized blasts with budded cytoplasm (black arrow), nuclear folding (yellow arrow), and some with Auer rods (red arrow). Pictures taken with oil at × 50 magnification. C Interphase nuclei showing one intact copy of RARα and two copies of the 5’ portion of the RARα probe. D Metaphases showing two 5’RARα signals hybridised to the der(17). E Isochromosome for the short arm of chromosome 17 and a derivative chromosome 17 which includes two copies of the long arms of chromosome 17. F Sanger sequencing of the STAT5b-RARα fusion. The Sanger sequencing at the breakpoint site is depicted by the black line
The patient subsequently tested negative for a PML::RARα rearrangement by both FISH (Fig. 1C) & RQ-PCR, following the protocol described by Europe Against Cancer (EAC). There was also no evidence of MECOM or KMT2A rearrangements. Chromosome analysis (Fig. 1E) of bone marrow cell cultures showed a karyotype consisting of 47 chromosomes including an isochromosome for the short arm of chromosome 17. There was also a derivative chromosome 17 which includes two copies of the long arms of this chromosome. ATRA treatment was discontinued at this point however subsequent FISH analysis, this time using an Vysis LSI RARα dual colour break-apart probe, displayed one intact copy of RARα along with two copies of the centromeric portion of the RARα probe. These copies were confirmed by metaphase FISH to be located on the der(17)—ATRA was therefore resumed pending further investigation. Three copies of TP53 were also identified. Based on karyotyping and FISH analysis, the results therefore represented a variant RARα rearrangement with an unknown cryptic partner gene.
In order to identify this cryptic partner, the sample was sent for myeloid next generation sequencing (NGS). DNA libraries were prepared using the KAPA Hyper Plus Kit (Kapa Biosystems) and sequenced using a Myeloid NGS assay covering common sequence, structural and copy number variations in genes involved in myeloid neoplasms. Alignment, de-duplication, and variant calling was performed using the MyeloidTS_WF bioinformatics pipeline v1.1. This panel uncovered a cryptic inv(17) or del(17)(q21.2q21.2) STAT5b e15 (NM_012448.4)::RARα e3 (NM_000964.4) variant. The sample was also sent to Newcastle Genetics laboratory, Newcastle upon Tyne, for an RNA fusion assay using the Illumina TruSight panel and confirmed by Sanger sequencing (Fig. 1F). Therefore, based on these genomic findings, the favoured classification according to the 2022 WHO classification is “APL with a variant RARα translocation” [1]. This is also in keeping with the International Consensus Classification [24].
The patients’ disease was judged to be resistant to ATO or ATRA, and due to extensive co-morbidities, he was not a candidate for intensive chemotherapy (ICT). Therefore, he was commenced on five cycles of low dose cytarabine (LDAC), 20 mg/m2 given subcutaneously once daily on days 1–10 of each 28 day cycle, beginning on cycle 1 day 1, and venetoclax (Ven) 100 mg from day 4 to day 28 orally with an azole after dose escalation from days 1–4. The bone marrow biopsy following cycle 1 was hypocellular with no excess of blasts/promyelocytes, and the patient was commenced on daily granulocyte colony-stimulating factor (G-CSF). Morphological remission (MR) was achieved at this point, demonstrating a clear sensitivity to a LDAC/Ven regimen. However, STAT5b::RARα mutant transcripts were still detectable by molecular minimal residual disease (MRD) and flow MRD was also positive. Cycle 2 was well tolerated. However, venetoclax dosage was reduced to 14 days as a consequence of prolonged cytopenias. Cytogenetic remission was achieved following this cycle. The patient proceeded with cycles three and four respectively with a delay in cycle five due to cytopenias and probable cellulitis. A subsequent bone marrow aspirate unfortunately revealed progressive disease morphologically (approximately 33% blasts) and continued MRD positivity. The patient was transferred to ICU with neutropenic sepsis with gram-positive cocci in culture and was treated with multiple broad spectrum antibiotics. The patient continued to deteriorate with persistent pyrexia and pancytopenia requiring transfusion support and died in ICU.
Discussion
This case highlights the complications that this variant of APL poses for both diagnosis and management. Consistent with other documented cases, this patient tested negative for a PML::RARα rearrangement by both FISH and RQ-PCR. Importantly, subsequent FISH testing used a RARα break apart probe that successfully identified the RARα 3’ deletion that often coincides with a STAT5b partner. The confirmation and precise identification of this cryptic RARα partner depended heavily on the custom NGS panel without which, identification of this partner would rely on the availability of a highly precise RQ-PCR assay or RNA sequencing [16]. Whilst the majority of commercially available DNA based myeloid NGS panels cannot detect structural variants and therefore will not exclude the presence of rare fusion genes such as that described in this case, the accessibility of this particular assay and its ability to detect rare structural variants, prompted specific confirmatory testing and swift application of the most suitable clinical management for this patient.
A comprehensive literature review of reported STAT5b::RARα positive APL cases, of which there were only 18, conducted by Zhang et al. [19] reported a median age at diagnosis of 39 ranging from 17 to 67 years of age and a greater propensity in the male population (15 patients were male and 3 were female). The majority of the treatment regimens employed across the 18 previous cases involved ICT using differing combinations of doxorubicin (DA), idarubicin (IA), fludarabine, mitoxantrone (MIT), fludarabine, cytarabine and G-CSF (FLAG), homoharringtone, and decitabine, achieving complete remission (CR) in 12 of the 18 patients. The median overall survival time was 9.5 months with a disease-free survival time of 3 months (Table 1). Whilst evidently ICT followed by HSCT is the preferred strategy utilised in patients with AML who are deemed fit, effective treatment options are, to date, extremely limited for those who are not suitable for HSCT. Our patient was ineligible for HSCT and ICT due to extensive co-morbidities.
Results from a phase 3 clinical trial conducted by Wei et al. [25] into the use of the combination LDAC/Ven in newly diagnosed AML, ineligible for ICT, demonstrated that this regime exhibits a well-tolerated safety profile and shows positive impacts on overall survival (OS), rates of remission, event free survival (EFS), transfusion requirements, and patient-reported outcomes compared with LDAC alone. This case represents, to the best of our knowledge, the first documented use of a LDAC/Ven regime in this extremely rare subtype of APL. Evidently, our patient showed a promising first-line response and sensitivity to this regime achieving MR after cycle 1. Further understanding of the mechanisms behind the anti-leukaemic activity and demonstrated disease sensitivity to a combined LDAC/Ven regime should be explored in an attempt to define a precise empirical treatment for this extremely rare subtype of APL, particularly in ineligible ICT candidates.
Although clearly effective for this patient, the use of LDAC/Ven is not the only therapy suitable for high-risk AML patients. Given the CD33 positivity of the present case the use of gemtuzumab ozogamicin (GO) may have been a potential option. GO is an anti-CD33 antibody conjugated to calicheamicin that has shown to be effective for the treatment of high-risk APL in recent clinical trials [26]. However, patients on this trial received ICT post-remission with a third of the recruited patients unable to complete prescribed regimens [3]. Unfortunately, this patient was not able to tolerate increased dosages or duration of therapy and deteriorated quickly before additional treatment strategies could be employed.
References
Khoury JD, Solary E, Abla O, et al (2022) The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia 36(7): 1703–1719. https://doi.org/10.1038/s41375-022-01613-1
Liquori A, Ibañez M, Sargas C, et al (2020) Acute promyelocytic leukemia: a constellation of molecular events around a single PML-RARA fusion gene. Cancers 12(3). https://doi.org/10.3390/cancers12030624
Iyer SG, Elias L, Stanchina M, Watts, J (2023) The treatment of acute promyelocytic leukemia in 2023: paradigm, advances, and future directions. Front Oncol 12. https://doi.org/10.3389/fonc.2022.1062524
Lo-Coco F, Avvisati G, Vignetti M and others (2013) Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med 369(2):111–121. https://doi.org/10.1056/NEJMoa1300874
Ciangola G, Gurnari C, Paterno G and others (2019) STAT5b-RARa-positive acute myeloid leukemia: diagnostic and therapeutic challenges of a rare AML subtype. Leuk Res 78:21–23. https://doi.org/10.1016/j.leukres.2019.01.004
Zhang G, Song Y, Wan L and others (2022) Treatment of STAT5b-RARA positive acute promyelocytic leukemia by venetoclax combining with homoharringtonine, cytarabine: a case report and literature review. Blood Sci 4(2):93–96. https://doi.org/10.1097/BS9.0000000000000111
Wen L, Xu Y, Yao L and others (2019) Clinical and molecular features of acute promyelocytic leukemia with variant retinoid acid receptor fusions. Haematologica 104(5):e195–e199. https://doi.org/10.3324/haematol.2018.205369
Arnould C, Philippe C, Bourdon V and others (1999) The signal transducer and activator of transcription STAT5b gene is a new partner of retinoic acid receptor in acute promyelocytic-like leukaemia. Hum Mol Genet 8(9):1741–1749. https://doi.org/10.1093/hmg/8.9.1741
Gallagher RE, Mak S, Paietta E and others (2004) Identification of a second acute promyelocytic leukemia (apl) patient with the STAT5b-RARα fusion gene among PML-RARα-Negative Eastern Cooperative Oncology Group (ECOG) APL Protocol Registrants. Blood 104(11):3005–3005. https://doi.org/10.1182/blood.V104.11.3005.3005
Kusakabe M, Suzukawa K, Nanmoku T and others (2008) Detection of the STAT5B–RARA fusion transcript in acute promyelocytic leukemia with the normal chromosome 17 on G-banding. Eur J Haematol 80(5):444–447. https://doi.org/10.1111/j.1600-0609.2008.01042.x
Iwanaga E, Nakamura M, Nanri T and others (2009) Acute promyelocytic leukemia harboring a STAT5B-RARA fusion gene and a G596V missense mutation in the STAT5B SH2 domain of the STAT5B-RARA. Eur J Haematol 83(5):499–501. https://doi.org/10.1111/j.1600-0609.2009.01324.x
Cahill TJ, Chowdhury O, Myerson SG, et al (2011) Myocardial infarction with intracardiac thrombosis as the presentation of acute promyelocytic leukemia. Circulation 123(10). https://doi.org/10.1161/CIRCULATIONAHA.110.986208
Qiao C, Zhang S, Chen L and others (2011) Identification of the STAT5B-RARα fusion transcript in an acute promyelocytic leukemia patient without FLT3, NPM1, c-Kit and C/EBPα mutation. Eur J Haematol 86(5):442–446. https://doi.org/10.1111/j.1600-0609.2011.01595.x
Chen H, Pan J, Yao L and others (2012) Acute promyelocytic leukemia with a STAT5b-RARα fusion transcript defined by array-CGH, FISH, and RT-PCR. Cancer Genet 205(6):327–331. https://doi.org/10.1016/j.cancergen.2012.02.007
Strehl S, König M, Boztug H and others (2013) All-trans retinoic acid and arsenic trioxide resistance of acute promyelocytic leukemia with the variant STAT5B-RARA fusion gene. Leukemia 27(7):1606–1610. https://doi.org/10.1038/leu.2012.371
Kluk MJ, Abo RP, Brown RD and others (2015) Myeloid neoplasm demonstrating a STAT5B-RARA rearrangement and genetic alterations associated with all- trans retinoic acid resistance identified by a custom next-generation sequencing assay. Mol Case Stud 1(1):a000307. https://doi.org/10.1101/mcs.a000307
Wang Y-Y, Hao J, Liu Z-Y and others (2015) Novel STAT5B–RARA fusion transcript in acute promyelocytic leukemia: identification and treatment response. Leuk Lymphoma 56(9):2731–2734. https://doi.org/10.3109/10428194.2015.1007454
Liu L, Chen S, Tan J and others (2016) Acute promyelocytic leukemia with stat5b- RARα fusiongene: a case report and literatures review. Zhonghua Xue Ye Xue Za Zhi = Zhonghua Xueyexue Zazhi 37(1):68–69. https://doi.org/10.3760/cma.j.issn.0253-2727.2016.01.014
Zhang C, Wang Y, Liu B and others (2018) Clinical characteristics of acute promyelocytic leukemia with the STAT5B-RARA fusion gene. Blood Cells Mol Dis 69:71–73. https://doi.org/10.1016/j.bcmd.2017.09.007
Pessina C, Basilico C, Genoni A and others (2017) A new acute myeloid leukemia case with STAT5B-RARA gene fusion due to 17q21.2 interstitial deletion. Leuk Lymphoma 58(8):1977–1980. https://doi.org/10.1080/10428194.2016.1262952
Wang A, Cai X, Qiang P, Duan Q (2018) Successful treatment of a patient with acute promyelocytic leukemia with a STAT5B / RARA fusion gene using decitabine. Leuk Lymphoma 59(3):763–765. https://doi.org/10.1080/10428194.2017.1357176
Peterson JF, He RR, Nayer H and others (2019) Characterization of a rarely reported STAT5B/RARA gene fusion in a young adult with newly diagnosed acute promyelocytic leukemia with resistance to ATRA therapy. Cancer Genet 237:51–54. https://doi.org/10.1016/j.cancergen.2019.06.007
Wang L, Yan X, He J (2020) Does acute promyelocytic leukemia patient with the STAT5B/RARa fusion gene respond well to decitabine?: A case report and literature review. Medicine 99(43):e22923. https://doi.org/10.1097/MD.0000000000022923
Arber DA, Orazi A, Hasserjian RP and others (2022) International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood 140(11):1200–1228. https://doi.org/10.1182/blood.2022015850
Wei AH, Montesinos P, Ivanov V and others (2020) Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood 135(24):2137–2145. https://doi.org/10.1182/blood.2020004856
Lancet JE, Moseley AB, Coutre SE and others (2020) A phase 2 study of ATRA, arsenic trioxide, and gemtuzumab ozogamicin in patients with high-risk APL (SWOG 0535). Blood Adv 4(8):1683–1689. https://doi.org/10.1182/bloodadvances.2019001278
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Patterson, J., Clarke, K., Mokretar, K. et al. Treatment of a STAT5b::RARα positive case of APL in a patient not eligible for intensive chemotherapy. Ir J Med Sci (2024). https://doi.org/10.1007/s11845-024-03751-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11845-024-03751-0