Abstract
Background
Maternally inherited non-syndromic hearing loss is linked with mitochondrial DNA mutations.
Aim
This investigation demonstrates the features of a Chinese pedigree suffering from maternally inherited non-syndromic hearing loss.
Methods
Biochemical characterizations included the measurements ofprotein synthesis levels, membrane potential, and the synthesis of reactive oxygen species (ROS) and adenosine triphosphate (ATP) using cybrid cell lines derived from an affected matrilineal subject and control subject.
Results
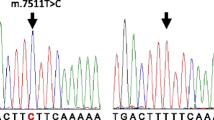
Non-congenital early or late-onset/development hearing impairment has been observed in 4 of 9 in a family (matrilineal), with different degrees of hearing impairment, ranging from normal to severe. A pedigree’s whole mitochondrial genome sequence analysis revealed the homoplasmic m.14502 T > C (I58V) mutation at ND6’s isoleucine location-58, and specific mitocchondrial DNA polymorphisms set haplogroups M10 were highly conserved. In vitro models indicated that m.14502 T > C mutation-derived respiratory deficiency decreases ND6 protein synthesis, mitochondrial membrane potential, and ATP synthesis. These mitochondrial dysregulations enhance the generation of ROS in the mutant cells. Identifying nuclear modifiers is essential for elucidating hearing loss’s pathogenesis and furnishing novel therapeutic interventions.
Conclusions
The m.14502 T > C mutation should be considered an inherited risk factor that can help diagnose. The data of this investigation help counsel families of individuals with hearing loss.







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Guan MX (2011) Mitochondrial 12S rRNA mutations associated with aminoglycoside ototoxicity. Mitochondrion 11:237–245
Zheng J, Ji Y, Guan MX (2012) Mitochondrial tRNA mutations associated with deafness. Mitochondrion 12:406–413
Ding Y, Leng J, Fan F et al (2013) The role of mitochondrial DNA mutations in hearing loss. Biochem Genet 51(7–8):588–602
Zhao H, Li R, Wang Q et al (2004) Maternally inherited aminoglycoside-induced and nonsyndromic deafness is associated with the novel C1494T mutation in the mitochondrial 12S rRNA gene in a large Chinese family. Am J Hum Genet 74:139–152
Yan X, Wang X, Wang Z et al (2011) Maternally transmitted late-onset non-syndromic deafness is associated with the novel heteroplasmic T12201C mutation in the mitochondrial tRNAHis gene. J Med Genet 48(10):682–690
Wang M, Liu H, Zheng J et al (2016) A deafness- and diabetes-associated tRNA mutation causes deficient pseudouridinylation at position 55 in tRNAGlu and mitochondrial dysfunction. J Biol Chem 291(40):21029–21041
Meng F, He Z, Tang X et al (2018) Contribution of the tRNAIle 4317A→G mutation to the phenotypic manifestation of the deafness-associated mitochondrial 12S rRNA 1555A→G mutation. J Biol Chem 293(9):3321–3334
Xue L, Chen Y, Tang X et al (2019) A deafness-associated mitochondrial DNA mutation altered the tRNASer(UCN) metabolism and mitochondrial function. Mitochondrion 46:370–379
Meng F, Zhou M, Xiao Y et al (2021) A deafness-associated tRNA mutation caused pleiotropic effects on the m1G37 modification, processing, stability and aminoacylation of tRNAIle and mitochondrial translation. Nucleic Acids Res 49(2):1075–1093
Bannwarth S, Abbassi M, Valéro R et al (2011) A novel unstable mutation in mitochondrial DNA responsible for maternally inherited diabetes and deafness. Diabetes Care 34(12):2591–2593
Lu J, Qian Y, Li Z et al (2010) Mitochondrial haplotypes may modulate the phenotypic manifestation of the deafness-associated 12S rRNA 1555A>G mutation. Mitochondrion 10(1):69–81
Andrews RM, Kubacka I, Chinnery PF et al (1999) Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet 23:147
Li R, Greinwald JH, Yang L et al (2004) Molecular analysis of mitochondrial 12S rRNA and tRNASer(UCN) genes in pediatric subjects with nonsyndromic hearing loss. J Med Genet 41:615–620
Kong QP, Bandelt HJ, Sun C et al (2006) Updating the East Asian mtDNA phylogeny: a prerequisite for the identification of pathogenic mutations. Hum Mol Genet 15:2076–2086
Ji Y, Liang M, Zhang J et al (2016) Mitochondrial ND1 variants in 1281 Chinese subjects with Leber’s hereditary optic neuropathy. Invest Ophthalmol Vis Sci 57(6):2377–2389
Meng F, Jia Z, Zheng J et al (2022) A deafness-associated mitochondrial DNA mutation caused pleiotropic effects on DNA replication and tRNA metabolism. Nucleic Acids Res 50(16):9453–9469
Ji Y, Nie Z, Meng F et al (2021) Mechanistic insights into mitochondrial tRNAAla 3’-end metabolism deficiency. J Biol Chem 297(1):100816
Ji Y, Zhang J, Lu Y et al (2020) Complex I mutations synergize to worsen the phenotypic expression of Leber’s hereditary optic neuropathy. J Biol Chem 295(38):13224–13238
Ji Y, Zhang J, Yu J et al (2019) Contribution of mitochondrial ND1 3394T>C mutation to the phenotypic manifestation of Leber’s hereditary optic neuropathy. Hum Mol Genet 28(9):1515–1529
Zhang S, Wang L, Hao Y et al (2008) T14484C and T14502C in the mitochondrial ND6 gene are associated with Leber’s hereditary optic neuropathy in a Chinese family. Mitochondrion 8(3):205–210
Zhao F, Guan M, Zhou X et al (2009) Leber’s hereditary optic neuropathy is associated with mitochondrial ND6 T14502C mutation. Biochem Biophys Res Commun 389(3):466–472
Ding Y, Zhang S, Guo Q, Zheng H (2022) Mitochondrial diabetes is associated with tRNALeu(UUR) A3243G and ND6 T14502C mutations. Diabetes Metab Syndr Obes 3(15):1687–1701
Chen H, Zheng J, Xue L et al (2012) The 12S rRNA A1555G mutation in the mitochondrial haplogroup D5a is responsible for maternally inherited hypertension and hearing loss in two Chinese pedigrees. Eur J Hum Genet 20(6):607–612
Zhang J, Zhou X, Zhou J et al (2010) Mitochondrial ND6 T14502C variant may modulate the phenotypic expression of LHON-associated G11778A mutation in four Chinese families. Biochem Biophys Res Commun 399(4):647–653
Funding
This study was funded by the Scientific Research fund of Taizhou Science and Technology Bureau (NO. 20ywb135) and Wenling Science and Technology Project (NO.2020S0180128; 2019S0180051; 2021S00152).
Author information
Authors and Affiliations
Contributions
Ting Zhang and Wenbin Wang designed the study. Ting Zhang, Renjie Su, and Wen Xiang acquired the data. Wenbin Wang wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
All authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, T., Su, R., Xiang, W. et al. Maternally inherited non-syndromic hearing loss is linked with a novel mitochondrial ND6 gene mutation. Ir J Med Sci 193, 937–943 (2024). https://doi.org/10.1007/s11845-023-03484-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-023-03484-6