Abstract
Background
Fatigue following acute viral illnesses is a major issue that complicates the clinical course of several epidemic and non-epidemic viral infections. There is a noticeably higher trend of patients with symptoms that persist after initial recovery from acute COVID-19. This study seeks to obtain more data about the prevalence of post-COVID-19 fatigue and the factors associated with higher fatigue frequency among patients who had COVID-19.
Methods
A single center cross-sectional study was performed between May 2021 and January 2022 at University Health, Kansas City, Missouri, USA. The Fatigue Assessment Scale (FAS) was utilized to measure post-COVID-19 fatigue. Descriptive and comparative statistics were used to describe clinical and sociodemographic features of patients. Analysis of variance (ANOVA), the chi-square test, and Fisher’s exact test were used to examine the statistical association between the FAS score and other clinical and sociodemographic factors.
Results
One hundred and fifty-seven patients who had been diagnosed with COVID-19 and diagnosed at University Health were enrolled in our study. Overall, 72% of patients (n = 113) were female. The mean ± standard deviation of the FAS score was 21.2 ± 9.0. The prevalence of post-COVID-19 fatigue among our studied sample was 43.3%. The findings of this study suggest that female patients have a significantly higher fatigue score compared with male patients (P < 0.05).
Conclusions
Post-COVID-19 fatigue is a major issue following the initial acute illness with COVID-19, with a prevalence of 43.3%. We recommend implementing standardized measures to screen for post-COVID-19 fatigue, especially among female patients.
Similar content being viewed by others
Background
The 2019 novel coronavirus (2019‐nCoV) is a new virus for which the initial clinically significant cases started in Wuhan, China. A few months later, the World Health Organization (WHO) declared 2019-nCoV as the causative agent of the new epidemic disease that was later named coronavirus disease 2019 (COVID‐19) [1]. This disease affects several body systems, most commonly the upper and lower respiratory tracts [2]. It also manifests with a wide variety of clinical symptoms, ranging from mild symptoms such as a sore throat, loss of taste and/or smell, to more severe symptoms, including cough, fever, shortness of breath, and multi-organ failure. Other symptoms include fatigue, myalgia, malaise, and diarrhea [3].
Fatigue is defined as a subjective feeling of tiredness, weariness, and lack of energy that exceeds physical exertion, and also negatively impacts a person’s ability to perform daily life activities [4]. In addition, fatigue is not alleviated by sleep or rest [5]. Fatigue is thought to be one of the most common symptoms affecting patients experiencing acute and chronic medical illnesses [6]. It not only affects physical health, but also significantly impacts other aspects including cognitive and psychological health [7]. It is also considered a major determinant that negatively impacts health-related quality of life, with more extreme and prolonged effects on this measure than other common symptoms including pain and nausea [8]. Fatigue can be classified as a symptom or a disease by itself, such as chronic fatigue syndrome [9].
Acute COVID-19 infection has been associated with fatigue, with a prevalence of about 36% [10, 11]. As the number of COVID-19 cases has increased dramatically since the beginning of the pandemic, there has been a noticeable increasing trend of patients with persistent symptoms past the acute phase. Some of these symptoms include loss of taste/smell, myalgia, chest tightness, non-restorative sleep, and most importantly, intense fatigue [12, 13]. The occurrence of these long-term adverse effects is concerning for a post-viral syndrome [13].
Post-viral fatigue has been reported in several epidemic and non-epidemic viral illnesses. During the 1918 Spanish flu pandemic, intense fatigue was one of the most common long-term symptoms complicating the post-flu period [14]. For the SARS epidemic in 2003 caused by SARS-CoV, Lam et al. [15] found that 40.3% of patients recovering from SARS reported chronic fatigue at the 4-year follow-up assessment. Magnus et al. [16] studied the H1N1 pandemic in 2009 in Norway and surprisingly found that individuals aged less than 30 years were more likely to develop chronic fatigue syndrome after the infection. For non-epidemic viruses, the well-studied cause of post-viral fatigue is Epstein–Barr virus (EBV), the causative agent of infectious mononucleosis, with up to 38% of patients experiencing persistent fatigue 2 months after disease onset [17].
Islam et al. [14] showed how common post-infectious fatigue is among several epidemic viral diseases (such as the Spanish flu and Ebola virus) and non-epidemic viral diseases (e.g., EBV). They clearly described their concern of post-viral fatigue following COVID-19, a view supported by recent surveys and social media postings. Giving these facts and the evolving COVID-19 pandemic, we carried out this study to determine the prevalence and severity of post-COVID-19 viral fatigue and to examine the factors that are associated with greater risk for developing this syndrome.
Methods
Study design
We performed this single-center observational cross-sectional study between May 2021 and January 2022.
Study setting
The study was conducted at University Health, Kansas City, Missouri; this is the primary teaching hospital for University of Missouri Kansas City (UMKC) School of Medicine. We used the medical records at University Health to gather information about the number of patients who had tested positive for COVID-19 during the study period.
Study population
The number of patients who had tested positive for COVID-19 at University Health between August 1, 2020, and March 1, 2021, was 1029; these patients comprised the study population.
Sample size
We used the Raosoft sample size web-based calculator to estimate our sample size, which was 157. We increased our sample size by 5% to cover patient non-response.
Participants and sampling technique
One hundred and fifty-seven patients were recruited. We set four inclusion criteria for our study:
-
1. ≥ 18 years old;
-
2. Patients who had tested positive for COVID-19 by nasopharyngeal polymerase chain reaction (PCR) at University Health between August 1, 2020 and March 1, 2021;
-
3. At least 4 weeks had passed since the initial diagnosis; and
-
4. A maximum of 6 months had passed since the initial diagnosis.
We excluded patients with cognitive restrictions that made independent responses to the questionnaire impossible. We also excluded patients with a prior diagnosis of fibromyalgia and/or chronic fatigue syndrome.
Data sources and variables
The questionnaire we implemented to gather data from patients contained two sections, one for sociodemographic information and the other for the validated Fatigue Assessment Scale (FAS). In the first section, we collected the following sociodemographic characteristics: the patient’s living arrangement (lives alone or with family), occupation, and smoking status. Data regarding other clinical and sociodemographic factors were obtained by reviewing the patient’s electronic medical records. Those were the patient’s gender, age, body mass index (BMI), hospitalization status (inpatient versus outpatient), and ethnicity. We categorized ethnicity as White, African American, and others. We also categorized BMI as underweight (< 18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (≥ 30 kg/m2). In the second section of the questionnaire, we included the FAS to assess the severity of fatigue in our studied sample. This scale is a standardized instrument for use as a measure of fatigue. It consists of 10 questions, each of which is answered by using a 5-point Likert-type scale ranging from 1 (“never”) to 5 (“always”). The scores on all questions except questions number 4 and 10 were recorded as Q1 = 1, Q2 = 2, Q3 = 3, Q4 = 4, and Q5 = 5. Questions 4 and 10 were reverse scored (Q1 = 5, Q2 = 4, Q3 = 3, Q4 = 2, and Q5 = 1). Michielsen and colleagues [18] developed this scale and analyzed its psychometric properties; they found an internal consistency of 0.9. The FAS total score ranges from 10, indicating the lowest level of fatigue, to 50, denoting the highest. An additional file was supplied to illustrate detailed description of the questionnaire (Additional file 1).
Data collection procedure
After selecting the patients who fit our inclusion criteria, the study team asked to de-identify the patients who met our inclusion criteria. We used the phone numbers recorded in the patients’ charts. The patients were called by one of the research group members; the calls were made Monday through Friday between 08:00 h and 16:00 h. The caller explained the reason for the call and the objectives of our study and offered enrollment in the study. Verbal consent was obtained from all the participants. The patients were asked the questions included in the questionnaire. The participant was considered a non-responder, if they did not respond twice on two different occasions. The calls were not recorded, and participation in the study was voluntary.
Ethical approval
The Institutional Review Board (IRB) of the University of Missouri Kansas City approved our study protocol. We also obtained verbal consent from each patient before including him/her in our study.
Statistical analysis
We entered our data and analyzed it by using R [19]. The data are expressed as the mean (standard deviation) or frequency (percentage). The FAS was the dependent variable. Clinical and sociodemographic factors were the independent variables. We used analysis of variance (ANOVA), the chi-square test, and Fisher’s exact test to examine the statistical association between FAS and other clinical and sociodemographic factors. The significance level was set at P < 0.05. We coded the independent variables such as gender, age, BMI, occupation, and ethnicity as dummy variables, with a value of 0, 1, or 2.
Results
Clinical and sociodemographic characteristics
A total of 157 patients who had been diagnosed with COVID-19 and diagnosed at University Health participated in this cross-sectional study. The number of patients who did not answer or refused to participate in our study was 205 patients. Overall, 72% (n = 113) of the patients were female and 28% (n = 44) were male. The majority of patients (84.1%, n = 132) were < 60 years old. Most of our patients (61.1%) were obese (BMI > 30 kg/m2). Most of the recruited sample (81.5%, n = 128) were living with their family, and 59.9% (n = 94) of patients in our sample were employed. The vast majority of patients were not smokers (75.8%, n = 119). Regarding ethnicity, 48.4% (n = 76) were African American, 38.2% (n = 60) are White, and 13.4% (n = 21) are of other ethnicities. Most of the patients in our sample (75.2%, n = 118) did not require hospitalization, while 24.8% (n = 39) had been admitted to the hospital. We interviewed the majority of our patients (79.6%, n = 125) 3 months or more from the COVID-19 diagnosis. Further details regarding the clinical and sociodemographic features of our studied sample are shown in Table 1.
Fatigue and health status
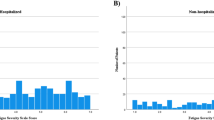
In patients who had been diagnosed with COVID-19 and diagnosed at University Health, the mean FAS score was 21.2 with a standard deviation (SD) of 9.0. The prevalence of fatigue in our studied sample was 43.3% (n = 68 patients); 12.1% (n = 19) of patients had extreme fatigue, denoted by an FAS score ≥ 35 (see Fig. 1). Table 2 shows the mean and standard deviation for each of the questions of the FAS.
Association between FAS scores and clinical and sociodemographic characteristics
The only variable that displayed a statistically significant association with the FAS score was gender (P < 0.05). On the other hand, the patient’s age, BMI, living status, occupation, ethnicity, smoking status, hospitalization status, and timing of interview were not significantly associated with the FAS (all Ps > 0.05). For more details regarding the association between clinical and sociodemographic variables and the FAS, see Table 3.
Discussion
We have evaluated fatigue among patients who had been diagnosed with COVID-19 and diagnosed at University Health. Although post-COVID-19 fatigue has been studied in other countries such as Saudi Arabia [20], Ireland [21], and India [22], to our knowledge our study is the first to evaluate post-COVID-19 fatigue among the US population. The FAS has been widely used to assess fatigue in different chronic medical conditions such as sarcoidosis [23], systemic lupus erythematosus (SLE) [24], and end-stage renal disease (ESRD) [25]. Our study is the second worldwide and the first in the USA to use this scale to assess post-COVID-19 fatigue; it was previously utilized by El Sayed et al. [20] in Saudi Arabia.
We found that the mean FAS score among patients who had been diagnosed with COVID-19 at our hospital was 21.2 (SD = 9.0). This is lower than the mean FAS score among patients with other chronic medical problems such as ESRD (mean 24.99) [25]. Chronic medical illnesses with significant morbidities can cause severe and more persistent fatigue compared with more acute illnesses such as post-viral fatigue; this might explain the lower percentage of fatigue among our study population [26]. The mean FAS score in our study is lower compared with a similar study of patients who had been diagnosed with COVID-19 in Saudi Arabia (40.81 ± 5.75) [20]. This goes against what has been reported in the literature, namely patients from more developed countries usually have higher fatigue prevalence [27, 28].
The prevalence of post-COVID 19 fatigue in our study was 43.3%. This is higher than the prevalence of post-infectious fatigue reported in other acute viral illness such as EBV (glandular fever), Coxiella burnetii (Q fever), or Ross River virus (epidemic polyarthritis) with the prevalence of 12%, as reported by Hickie et al. [29] in their prospective cohort study. The prevalence in this study is also higher than the prevalence of post-viral fatigue of 28% that resulted from Ebola virus infection [30]. Townsend et al. [21] also found a significant burden of fatigue among patients who had been diagnosed with COVID-19 in Ireland, with a 52.3% prevalence of post-COVID-19 fatigue, which is similar to what we found. These findings clearly indicate that COVID-19 is one of the major causes of post-viral fatigue compared with other epidemic and non-epidemic viral illness.
Our study demonstrated that female patients have significantly higher FAS scores compared with male patients. Other researchers have reported similar results of higher fatigue frequency in women in other chronic medical illnesses. For example, Mollaoğlu and Üstün [31] found that gender is one of the major determinants of fatigue severity in patients with multiple sclerosis. In patients with cancer, the level of fatigue, anxiety, and depression was also higher in women [32]. In addition, women were significantly more likely to report post-infectious fatigue in other acute viral infections such as West-Nile virus infection [33]. Female gender has also been associated with higher risk for the development of chronic fatigue syndrome after infectious mononucleosis in adolescents [34]. El Sayed et al. [20] studied post-COVID-19 fatigue and found a significant positive correlation between gender and FAS score (P < 0.05). However, they found higher fatigue scores in men (mean FAS in men = 41.5, mean FAS in women = 38.89). Another study on patients who had been diagnosed with COVID-19 in Ireland showed a distinct female preponderance in the development of fatigue after acute COVID-19 infection [21], a finding that is consistent with our study. This can be explained because, in addition to employment, women have other social duties, including taking care of young children; these additional responsibilities might increase their chances of developing post-infection fatigue [35]. The mean age of the female population in this study was 44.4 years. With advancing age, women might have an increased risk of fatigue attributed to menstrual abnormalities, early menopause, endometriosis, pelvic pain, and hysterectomy [36], which we did not account for in our study.
It is unclear whether viral illnesses can cause or contribute to the development of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) [37]. Given the growing number of COVID-19 cases, the emergence of new variants and the persistence of symptoms in a sizable proportion of patients, development of ME/CFS might be a concern in this pandemic. More long-term studies need to be conducted to assess this possible association.
Strengths and limitations
Notwithstanding the relatively limited sample size, this work has many positive points. For example, to our knowledge it is the first study to assess post-COVID 19 fatigue in the US population. Moreover, it is the second study worldwide and the first in the USA to use the FAS to determine the severity of fatigue in patients who had been diagnosed with COVID-19. In addition, we included patients from multiple races given the multiracial nature of our hospital population. On the other hand, there are some limitations we have to mention. First, our study is cross-sectional in nature, so we could not identify a cause-effect relationship and only assessed participants at a single time point. Second, the absence of control groups (i.e., patients with other viral infections such as infectious mononucleosis or influenza) limits the interpretation of the disease burden and its association with fatigue. Furthermore, there are some clinical and social determinants that might affect post-viral fatigue that we did not include in our study, such as educational level, monthly income, and some routine laboratory measures. Moreover, there are concerns for recall bias in the studied sample as participants who agreed to participate in the study were more likely to report fatigue compared with individuals that declined to participate in the study. Lastly, our sample included more women (72%) than men, and this might result in a selection bias.
Conclusion
Following the initial acute illness with COVID-19, post-COVID-19 fatigue seems to be a major health concern, with a prevalence of 43.3% in our study population. This is noticeably higher than post-viral fatigue found in other epidemic and non-epidemic viral infections. Women had significantly higher fatigue in the period following the initial acute illness with COVID-19 compared with men. This study highlights the burden of post-COVID-19 fatigue. Therefore, we recommend implementing standardized methods in clinical practice to screen for post-COVID-19 fatigue so as to apply early interventions to help patients who experience significant fatigue. We also recommend that health care providers give more attention to female patients following acute COVID-19 illness, because they are at significantly higher risk of developing post-COVID-19 fatigue.
References
Sun P, Lu X, Xu C et al (2020) Understanding of COVID-19 based on current evidence. J Med Virol 92(6):548–551. https://doi.org/10.1002/jmv.25722
Lovato A, de Filippis C, Marioni G (2020) Upper airway symptoms in coronavirus disease 2019 (COVID-19). Am J Otolaryngol 41(3):102474. https://doi.org/10.1016/j.amjoto.2020.102474
Esakandari H, Nabi-Afjadi M, Fakkari-Afjadi J et al (2020) A comprehensive review of COVID-19 characteristics. Biol Proced Online 22:19. https://doi.org/10.1186/s12575-020-00128-2
Nocerino A, Nguyen A, Agrawal M et al (2020) Fatigue in inflammatory bowel diseases: etiologies and management. Adv Ther 37(1):97–112. https://doi.org/10.1007/s12325-019-01151-w
Czuber-Dochan W, Norton C, Bassett P et al (2014) Development and psychometric testing of inflammatory bowel disease fatigue (IBD-F) patient self-assessment scale. J Crohns Colitis 8(11):1398–1406. https://doi.org/10.1016/j.crohns.2014.04.013
Ream E, Richardson A (1996) Fatigue: a concept analysis. Int J Nurs Stud 33(5):519–529. https://doi.org/10.1016/0020-7489(96)00004-1
McCabe M (2009) Fatigue in children with long-term conditions: an evolutionary concept analysis. J Adv Nurs 65(8):1735–1745. https://doi.org/10.1111/j.1365-2648.2009.05046.x
Curt GA (2000) Impact of fatigue on quality of life in oncology patients. Semin Hematol 37(4 Suppl 6):14–17. https://doi.org/10.1016/s0037-1963(00)90063-5
Flechtner H, Bottomley A (2003) Fatigue and quality of life: lessons from the real world. Oncologist 8(Suppl 1):5–9. https://doi.org/10.1634/theoncologist.8-suppl_1-5
Li LQ, Huang T, Wang YQ et al (2020) COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol 92(6):577–583. https://doi.org/10.1002/jmv.25757
Del Rio C, Malani PN (2020) COVID-19—new insights on a rapidly changing epidemic. JAMA 323(14):1339–1340
Davido B, Seang S, Tubiana R, de Truchis P (2020) Post-COVID-19 chronic symptoms: a postinfectious entity? Clin Microbiol Infect 26(11):1448–1449. https://doi.org/10.1016/j.cmi.2020.07.028
Perrin R et al (2020) Into the looking glass: post-viral syndrome post COVID-19. Med Hypotheses144:110055
Islam MF, Cotler J, Jason LA (2020) Post-viral fatigue and COVID-19: lessons from past epidemics. Fatigue Biomed Health Behav 8(2):61–69
Lam MH, Wing YK, Yu MW et al (2009) Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med 169(22):2142–2147. https://doi.org/10.1001/archinternmed.2009.384
Magnus P et al (2015) Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) is associated with pandemic influenza infection, but not with an adjuvanted pandemic influenza vaccine. Vaccine 33(46):6173–6177
Buchwald DS et al (2000) Acute infectious mononucleosis: characteristics of patients who report failure to recover. Am J Med 109(7):531–537
Michielsen HJ, De Vries J, Van Heck GL (2003) Psychometric qualities of a brief self-rated fatigue measure the fatigue assessment scale. J Psychosomatic Res 54:345–352
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
El Sayed S, Shokry D, Gomaa SM (2021) Post-COVID-19 fatigue and anhedonia: a cross-sectional study and their correlation to post-recovery period. Neuropsychopharmacol Rep 41(1):50–55. https://doi.org/10.1002/npr2.12154
Townsend L, Dyer AH, Jones K et al (2020) Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE 15(11):e0240784. https://doi.org/10.1371/journal.pone.0240784
Mittal J, Ghosh A, Bhatt SP et al (2021) High prevalence of post COVID-19 fatigue in patients with type 2 diabetes: a case-control study. Diabetes Metab Syndr 15(6)
Hendriks C, Drent M, Elfferich M, De Vries J (2018) The Fatigue Assessment Scale: quality and availability in sarcoidosis and other diseases. Curr Opin Pulm Med 24(5):495–503. https://doi.org/10.1097/MCP.0000000000000496
Horisberger A, Courvoisier D, Ribi C (2019) The Fatigue Assessment Scale as a simple and reliable tool in systemic lupus erythematosus: a cross-sectional study. Arthritis Res Ther 21(1):80. https://doi.org/10.1186/s13075-019-1864-4
Zyga S, Alikari V, Sachlas A et al (2015) Assessment of fatigue in end stage renal disease patients undergoing hemodialysis: prevalence and associated factors. Med Arch 69(6):376–380. https://doi.org/10.5455/medarh.2015.69.376-380
Finsterer J, Mahjoub SZ (2014) Fatigue in healthy and diseased individuals. Am J Hosp Palliat Care 31(5):562–575. https://doi.org/10.1177/1049909113494748
Hifinger M, Putrik P, Ramiro S et al (2016) In rheumatoid arthritis, country of residence has an important influence on fatigue: results from the multinational COMORA study. Rheumatology (Oxford) 55(4):735–744. https://doi.org/10.1093/rheumatology/kev395
Skapinakis P, Lewis G, Mavreas V (2003) Cross-cultural differences in the epidemiology of unexplained fatigue syndromes in primary care. Br J Psychiatry 182:205–209. https://doi.org/10.1192/bjp.182.3.205
Hickie I, Davenport T, Wakefield D et al (2006) Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ 333(7568):575. https://doi.org/10.1136/bmj.38933.585764.AE
Wilson HW, Amo-Addae M, Kenu E et al (2018) Post-Ebola syndrome among Ebola virus disease survivors in Montserrado County, Liberia 2016. Biomed Res Int 2018:1909410
Mollaoğlu M, Üstün E (2006) Fatigue in multiple sclerosis patients. J Clin Nurs 18(9):1231–1238
Tel H, Tel H, Doğan S (2011) Fatigue, anxiety and depression in cancer patients. Neurol Psychiatry Brain Res 17(2):42–45
Garcia MN, Hause AM, Walker CM et al (2014) Evaluation of prolonged fatigue post–West Nile virus infection and association of fatigue with elevated antiviral and proinflammatory cytokines. Viral Immunol 27(7):327–333
Katz BZ, Shiraishi Y, Mears CJ et al (2009) Chronic fatigue syndrome after infectious mononucleosis in adolescents. Pediatrics 124(1):189–193
Bensing JM, Hulsman RL, Schreurs KM (1999) Gender differences in fatigue: biopsychosocial factors relating to fatigue in men and women. Med Care 1078–1083
Boneva RS, Lin JM, Unger ER (2015) Early menopause and other gynecologic risk indicators for chronic fatigue syndrome in women. Menopause 22(8):826–834. https://doi.org/10.1097/GME.0000000000000411
Rasa S, Nora-Krukle Z, Henning N et al (2018) Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Transl Med 16(1):268. https://doi.org/10.1186/s12967-018-1644-y
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khatib, S., Sabobeh, T., Habib, A. et al. Post-COVID-19 fatigue as a major health problem: a cross-sectional study from Missouri, USA. Ir J Med Sci 192, 699–705 (2023). https://doi.org/10.1007/s11845-022-03011-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-022-03011-z