Abstract’
Introduction/aims
There are disparities in the availability of systemic anticancer therapies (SACTs) globally. We set out to investigate the cost and reimbursement of SACTs in the United Kingdom (UK) and the Republic of Ireland (ROI) in conjunction with efficacy and licensing authority decisions in the United States (US) and the European Union (EU).
Methods
We sought data pertaining to licensing in the EU, reimbursement in ROI/UK and cost/efficacy of SACTs licensed by the Food and Drug Administration (FDA) between January 2015 and May 2021. Independent samples t tests, chi-square test and Pearson’s correlation were used for statistical analysis.
Results
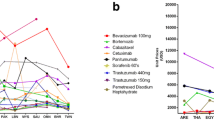
We identified that the majority of FDA-approved regimens are licensed by the European Medicines Agency (EMA) (n = 91, 67.9%). However, only a minority of these are currently reimbursed in the UK (n = 60, 45%) or the ROI (n = 28, 21%) as of the 1st of May 2021. In addition, only a minority of regimens have demonstrated a statistically significant OS benefit (n = 54, 40%). There was no association between cost of regimens and either the presence (t = 0.846, p = 0.40) or duration of OS benefit (t = − 0.84, p = 0.64).
Conclusions
Our study highlights that many licensed systemic anticancer treatments are not currently reimbursed in ROI/UK. The high cost of these medicines is independent of the presence of an OS benefit. Collaboration between regulatory agencies, governments and industry partners is needed to ensure health expenditure is directed towards the most effective treatments.




Similar content being viewed by others
Change history
29 April 2022
The author family name has been change to O'Reilly to correct the citation online.
Abbreviations
- ADC:
-
Antibody-drug conjugates
- BRAF:
-
B-Raf proto-oncogene
- CDF:
-
Cancer Drugs Fund
- EMA:
-
European Medicines Agency
- DOH:
-
Department of Health
- FDA:
-
Food and Drug Association
- GDP:
-
Gross domestic product
- HSE:
-
Health Service Executive
- ICI:
-
Immune checkpoint inhibitors
- IPHA:
-
Irish Pharmaceutical Healthcare Association
- NCPE:
-
National Centre for Pharmacoeconomics
- NHS:
-
National Health Service
- NICE:
-
National Institute for Clinical Excellence
- ORR:
-
Overall response rate
- OS:
-
Overall survival
- pCR:
-
Pathological complete response
- PFS:
-
Progression-free survival
- QOL:
-
Quality of life
- R&D:
-
Research and development
- RCT:
-
Randomised controlled trials
- ROI:
-
Republic of Ireland
- SACT:
-
Systemic anticancer treatment
- TKI:
-
Tyrosine kinase inhibitor
- UK:
-
United Kingdom
- US:
-
United States
References
Food and Drugs Association (2020) Drug approvals and databases. p. 1. Available from: https://www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases
Jackson K, Nahata MC (2017) Rising cost of anticancer medications in the United States. Ann Pharmacother 51(8):706–10. Available from: https://doi.org/10.1177/1060028017702406
Xu K, Soucat A, Kutzin J, Brindley C (2018) Public spending on health : a closer look at global trends. Geneva. Available from: https://apps.who.int/iris/bitstream/handle/10665/276728/WHO-HIS-HGF-HF-WorkingPaper-18.3-eng.pdf?ua=1
Belloni A, Morgan D (2018) Pharmaceutical expenditure and policies : past trends and future challenges. OECD (87):0–75
Martinalbo J, Bowen D, Camarero J et al (2016) Early market access of cancer drugs in the EU. Ann Oncol 27(1):96–105. Available from: https://doi.org/10.1093/annonc/mdv506
Johnson JR, Ning Y-M, Farrell A, Justice R, Keegan P, Pazdur R (2011) Accelerated approval of oncology products: the food and drug administration experience. J Natl Cancer Inst 103(8):636–644
Wilking N, Bucsics A, Kandolf Sekulovic L et al (2019) Achieving equal and timely access to innovative anticancer drugs in the European Union (EU): summary of a multidisciplinary CECOG-driven roundtable discussion with a focus on Eastern and South-Eastern EU countries. ESMO Open 4(6):e000550
Zhang Y, Hueser HC, Hernandez I (2017) Comparing the approval and coverage decisions of new oncology drugs in the United States and other selected countries. J Manag care Spec Pharm 23(2):247–254
Oireactas Eireann (2013) Health (pricing and supply of medical good) Act 2013. Ireland. Available from: http://www.irishstatutebook.ie/eli/2013/act/14/enacted/en/html
Health D (2012) Framework agreement on the supply and pricing of medicines. Dublin 2012. Available from: https://www.hse.ie/eng/about/who/cpu/iphaagreement2016.pdf
Food and Drug Administration (2020) Hematology/Oncology (Cancer) Approvals & Safety Notifications. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/hematologyoncology-cancer-approvals-safety-notifications
EMA (2020) European Medicines Agency. Available from: https://www.ema.europa.eu/en
Association F and D (2021) Oncology/heamatology malignancies approval notifications. p. 1. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/oncology-cancer-hematologic-malignancies-approval-notifications
Health Service Executive (2020) List of high tech items as of 1st October 2020. p. 1. Available from: https://www.hse.ie/eng/staff/pcrs/online-services/august-2020-high-tech-list.pdf
Wilson MK, Collyar D, Chingos DT, Friedlander M, Ho TW, Karakasis K et al (2015) Outcomes and endpoints in cancer trials: bridging the divide. Lancet Oncol 16(1):e43-52
Prasad V, Mailankody S (2017) Research and development spending to bring a single cancer drug to market and revenues after approval. JAMA Intern Med 177(11):1569–75. Available from: https://doi.org/10.1001/jamainternmed.2017.3601
Collier R (2009) Drug development cost estimates hard to swallow. Can Med Assoc J 180(3):279 LP – 280. Available from: http://www.cmaj.ca/content/180/3/279.abstract
Ledley FD, McCoy SS, Vaughan G, Cleary EG (2020) Profitability of large pharmaceutical companies compared with other large public companies. JAMA 323(9):834–843
Lozano-Blázquez A, Dickson R, Fraga-Fuentes M-D, Martínez-Martínez F, Calleja-Hernández M-Á (2015) Differences in cancer drug assessment between Spain and the United Kingdom. Eur J Cancer 51(13):1843–1852
Morrell L, Wordsworth S, Fu H et al (2017) Cancer drug funding decisions in Scotland: impact of new end-of-life, orphan and ultra-orphan processes. BMC Health Serv Res 17(1):613. Available from: https://doi.org/10.1186/s12913-017-2561-0
Grover P, Babar Z-U-D, Oehmen R, Vitry A (2018) Medicines access programs to cancer medicines in Australia and New Zealand: An exploratory study. Health Policy (3):243–9.
van de Vooren K, Curto A, Freemantle N, Garattini L (2015) Market-access agreements for anti-cancer drugs. J R Soc Med 108(5):166–170
Vitry A, Mintzes B, Lipworth W (2016) Access to new cancer medicines in Australia: dispelling the myths and informing a public debate. J Pharm policy Pract 9:13. Available from: https://pubmed.ncbi.nlm.nih.gov/27057313
Saluja R, Arciero VS, Cheng S et al (2018) Examining trends in cost and clinical benefit of novel anticancer drugs over time. J Oncol Pract 14(5):e280–e294
Chamberlain C, Collin SM, Stephens P et al (2014) Does the cancer drugs fund lead to faster uptake of cost-effective drugs? A time-trend analysis comparing England and Wales. Br J Cancer 111(9):1693–702. Available from: https://pubmed.ncbi.nlm.nih.gov/24569469
McCabe C, Paul A, Fell G, Paulden M (2016) Cancer drugs fund 2.0: a missed opportunity? Pharmacoeconomics 34(7):629–33
Ocana A, Amir E, Tannock IF (2016) Toward value-based pricing to boost cancer research and innovation. Cancer Res 76(11):3127 LP – 3129. Available from: http://cancerres.aacrjournals.org/content/76/11/3127.abstract
Cherny NI, Sullivan R, Dafni U et al (2016) Magnitude of Clinical Benefit Scale vol 1.0 questions and answers ESMO Open 1(5) Available from: https://doi.org/10.1136/esmoopen-2016-000100
Network NCC (2022) NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) with NCCN Evidence Blocks. United States. Available from: https://www.nccn.org/guidelines/guidelines-with-evidence-blocks
Cortes J, Perez-García JM, Llombart-Cussac A et al (2020) Enhancing global access to cancer medicines. CA Cancer J Clin (2):105–24. Available from: https://doi.org/10.3322/caac.21597
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
As per our local ethical guidelines, ethical approval was not required for this study.
Conflict of interest
DOR has received educational grants from Pfizer and Servier. RB has received educational grants from Pfizer, Astellas and Jannsen. He is co-founder of Portable Medical Technology. SOR has received consultant fees from Astrazeneca, travel expenses from Astrazeneca, Pfizer, Novartis, Roche and honoraria from Novartis, Roche, Pfizer. DCC has received consultant fees from Genmab, MSD; research funding from Pfizer; travel expenses from Roche, MSD, Genmab; and honoraria from Amgen, Pfizer, Roche, MSD. RC has received an unrestricted educational grant from Pfizer. SN is a former employee of MSD and has received honoraria from Pfizer and meeting registration expenses from Pfizer, MSD and Roche.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
O’Reilly, D., McLaughlin, R., Ronayne, C. et al. Cost and public reimbursement of cancer medicines in the UK and the Republic of Ireland. Ir J Med Sci 192, 541–548 (2023). https://doi.org/10.1007/s11845-022-02990-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-022-02990-3