Abstract
The World Health Organisation (WHO) End Tuberculosis (TB) Strategy and the WHO Framework Towards Tuberculosis Elimination in Low Incidence Countries state that latent tuberculosis infection (LTBI) screening and treatment in selected high-risk groups is a priority action to eliminate TB. The European Centre for Disease Prevention and Control (ECDC) advises that this should be done through high-quality programmatic management, which they describe as having six key components. The research aim was to systematically review the literature to identify what is known about the epidemiology of LTBI and the uptake and completion of LTBI screening and treatment in Ireland to inform the programmatic management of LTBI nationally. A systematic literature review was performed according to a review protocol and reported in adherence with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement. Twenty-eight studies were eligible for inclusion and described LTBI screening or treatment performed in one of five contexts, pre-biologic or other immunosuppression screening, people living with HIV, TB case contacts, other vulnerable populations, or healthcare workers. The risk of bias across studies with regard to prevalence of LTBI was generally high. One study reported a complete cascade of LTBI care from screening initiation to treatment completion. This systematic review has described what published research there is on the epidemiology and cascade of LTBI care in Ireland and identified knowledge gaps. A strategy for addressing these knowledge gaps has been proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reactivation of latent tuberculosis (TB) infection (LTBI) is a significant challenge for global TB elimination efforts. It is estimated that 23% of the world’s population and 13.7% of Europe’s population have LTBI [1]. The World Health Organisation’s (WHO) End TB Strategy and Framework Towards Tuberculosis Elimination in Low-Incidence Countries state that LTBI screening and treatment in selected high-risk groups are priority actions to eliminate TB [2, 3]. The European Centre for Disease Prevention and Control (ECDC) advises that this should be done through high-quality programmatic management, which they describe as having six key components (Table 1) [4].
Risk groups with a high prevalence of LTBI or a high risk of TB reactivation should be prioritised for LTBI screening and treatment [2,3,4]. For some cohorts, whether programmatic LTBI screening and treatment occurs depends on the country-specific epidemiology of LTBI and the resources available for screening and treatment (Table 2) [3, 4]. As well as identifying cohorts who should be screened and treated for LTBI, it is important to know whether programmatic LTBI management in these cohorts is feasible by having prior knowledge of the uptake and completion of LTBI screening and treatment (known as the cascade of care) and having considered its cost and cost-effectiveness [4].
Many countries with a low incidence of TB are establishing programmatic LTBI management to achieve TB elimination after researching the prevalence of LTBI in different cohorts and the feasibility of programmatic LTBI management. In the United Kingdom (UK), they have identified that immigrants from countries with a very high incidence of TB contribute significantly to the case burden nationally [5, 6]. They have demonstrated a high prevalence of LTBI among these immigrant cohorts and demonstrated that the rate of TB reactivation over time was significant, suggesting that TB reactivation, as opposed to primary TB infection, explained the high TB incidence in this cohort [7, 8]. Furthermore, they have researched the feasibility, acceptability and cost effectiveness of different screening strategies among high-risk immigrant cohorts [9,10,11,12]. Public Health England has established a national LTBI testing and treatment program for immigrants from countries with a high incidence of TB informed by their research on the prevalence of LTBI and feasibility of programmatic screening in this cohort [13]. This was a key action of their national collaborative strategy for TB [14]. Evidently, LTBI epidemiological and cascade of care research informed and enabled Public Health England to establish programmatic LTBI management in a target risk cohort.
The aim of this systematic review was to identify what is known about the epidemiology and cascade of care of LTBI in Ireland to inform its programmatic management nationally.
Methods
A systematic literature review was performed according to a review protocol and reported in adherence with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (Appendix 1) [15]. The protocol for this systematic review was registered with the Open Science Framework (https://doi.org/10.17605/OSF.IO/8ED29) and is available in Appendix 2.
Studies eligible for inclusion were those that described any group of patients who were screened or treated for LTBI in Ireland and reported using any one or a combination of chest radiography, tuberculin skin resting (TST) or interferon-gamma release assay (IGRA) testing to screen for LTBI. Studies had to report at least one of the following outcomes (chosen because they describe the cascade of LTBI care): the proportion of people screened out of the target population, the prevalence of a positive screening test in the target population, the proportion of those diagnosed with LTBI who were offered treatment, the proportion of those diagnosed with LTBI who started treatment for LTBI, the proportion of those diagnosed with LTBI who completed treatment out and the cost of performing screening or treatment of LTBI cases identified.
Clinical audits, randomized controlled trials, diagnostic accuracy studies, retrospective cohort reviews and prospective cohort reviews published between the 1st of January 2000 and the 31st of December 2019 (inclusive) were eligible for inclusion. Studies published in languages other than English were not eligible for inclusion. Studies where it was not possible to extract data on patients screened in Ireland alone were excluded.
A search of MEDLINE (via OVID), Embase, Web of Science, Google Scholar and published abstracts from national conferences in Ireland was conducted (search strategy is described in Appendix 2, date of last search: 14th of May 2020). The references of included studies were also searched. The literature search and data extraction were each conducted independently by two reviewers, and any disagreements relating to study eligibility or data extraction were resolved by discussion and mutual agreement. For the prevalence of a positive screening test, the risk of bias was assessed using a tool designed for TB prevalence studies that was derived from on an existing tool for prevalence studies (Appendix 3) [16].
Results
Search results
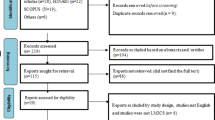
The results of the search are described in Fig. 1. Fifty-two articles were identified for full-text review from the search of the indexed literature, Google Scholar, conference abstract searches, and the references of included articles. In total, 28 studies were identified as meeting the review inclusion criteria.
Characteristics of included studies
The included studies described LTBI screening or treatment performed in one of five contexts (Table 3), pre-biologic or other immunosuppressive treatment screening (11 studies [17,18,19,20,21,22,23,24,25,26,27]), people living with human immunodeficiency virus (PLWHIV) (two studies [28, 29]), TB case contacts or prior to Bacillus Calmette–Guérin (BCG) vaccination (nine studies [30,31,32,33,34,35,36,37,38]), other vulnerable populations (two studies in asylum seekers [39, 40]) or health care workers (five studies [29, 41,42,43,44]). Most studies (19/28) were retrospective cohort reviews, seven were prospective cohort reviews and for one study, the design was unclear. All studies were performed on a regional or local level. Fourteen of 28 studies were conducted in tertiary care centres, nine were conducted in public health departments, and three were in secondary care centres.
The reporting of the cascade of care was generally very incomplete (Table 4). Only six studies described the proportion of the target population that completed screening. Provider recommendation, patient acceptance and patient completion of LTBI treatment were reported in 10, 12 and eight studies, respectively. One of 28 studies reported the complete cascade of care from screening initiation to treatment completion [17]. One study reported an estimate of the cost of LTBI screening and treatment [26].
Risk of bias assessment
The overall risk of bias in assessing the prevalence of LTBI in the included studies was high (Table 5, Appendix 3). Convenience sampling occurred in 25 of 26 studies. In 12 of 26 studies, there was a lack of a description of the patient exclusion criteria or how TB disease was identified. In 19 of 26 studies, the response rate, or the proportion of the target population who were screened, was not reported. Overall, the risk of bias was low (score 6–8) in two studies, moderate (score 3–5) in seven studies and high (score 0–2) in 17 studies.
In the 11 studies evaluating LTBI screening in immunosuppressed patients, the risk of bias was high in six of 11 studies, moderate in four of 11 studies and low in one of 11 studies. In the two studies evaluating LTBI screening in people living with HIV, the risk of bias was high in one study and moderate in the other. In the eight studies evaluating LTBI screening in recent TB contacts, the risk was high in six of eight studies, moderate in one of eight studies and low in one of eight studies. In the two studies evaluating LTBI screening in asylum seekers, the risk of bias was moderate in one study and low in the other. All four studies which evaluated LTBI screening in health care workers had a high risk of bias. Aside from the risk of bias within studies, there is a risk of reporting bias across studies. Studies where any one or more of the offering, uptake and completion of screening or treatment for LTBI was poor may not have reported these outcomes.
Latent TB infection screening and treatment outcomes in patients undergoing immunosuppression
Gnanasekaran et al. [17] was the only study that reported the proportion of the target population screened (95% of the target cohort) (Table 6). The median prevalence of a positive IGRA across all studies in this cohort was 7% (interquartile range (IQR) 7–8%). When considering only the studies where the risk of bias was moderate-low, the prevalence of a positive IGRA was 7% (IQR 5–7%). The median prevalence of a positive TST across all studies in this cohort was 17% (13–26%) and when considering only studies where the risk of bias was moderate-low, the median prevalence of a positive TST was 17% (IQR 15–18%).
In all seven studies where the proportion of patients who were offered and accepted LTBI treatment was reported [17,18,19,20, 26, 27, 46], all patients were offered and accepted treatment. The median proportion of patients completing treatment was 100% (IQR 90–100%), with all patients in three studies [17, 19, 26] completing treatment, and 61% of patients in one study [23] completing treatment. Jordan et al. [26] reported the cost of treating four patients with LTBI diagnosed using an IGRA as €1652 and 21 patients diagnosed using a TST as €6174, although the methodology used to make these cost estimates is unclear.
Latent TB infection screening and treatment outcomes in people living with HIV
Ni Cheallaigh et al. [28] reported the proportion of people living with HIV who had a positive test when screened using an IGRA as 18% when T-SPOT was used and 11% when QuantiFERON was used (sample sizes 256 and 247, respectively). When a TST was used among PLWHIV, the proportion of patients diagnosed with LTBI was 10% in the study by Ni Cheallaigh et al. [28] and 11% in the study by Ali et al. [29] (sample sizes 93 and 331 respectively). However, the risk of bias in the study by Ali et al. [29] was high. Ni Cheallaigh et al. [28] reported that all patients who were diagnosed with LTBI were offered treatment. No study reported on the proportion of patients completing LTBI treatment in this cohort.
Latent TB infection screening and treatment outcomes in recent TB contacts or prior to BCG vaccination
Three studies reported on the proportion of the target sample screened as part of contact tracing with 97%, 83% and 79% respectively being screened (Table 7) [30, 31, 33, 35]. Seven studies reported on the proportion of patients diagnosed with LTBI in this cohort [30,31,32,33,34,35,36]. There were only two studies where the risk of bias was moderate-low and the screening test used was reported in the context of TB contact tracing. The study by O’Meara et al. described a TB outbreak in a primary school setting with 191 children screened using a TST [33]. Gaensbaeur et al. reported on two TB outbreaks in creches where 268 children were screened [36]. The prevalence of a positive TST in these studies was 9% and 7% respectively. One study reported on the proportion of recent TB contacts diagnosed with LTBI who were offered treatment, 61% [31]. Two studies reported on the proportion of TB case contacts accepting treatment as 31% and 67% [30, 35]. Two studies reported on the proportion of patients completing treatment as 33% and 77% [30, 35]. Two studies described the outcome of LTBI screening prior to BCG vaccination, one of which reported 13 cases of LTBI being offered treatment, 10 of whom accepted and completed treatment [38].
Latent TB infection screening and treatment outcomes in asylum seekers
Millar et al. [39] reported on the proportion of the target sample screened in asylum seekers (28%) where screening was voluntary. Doyle et al. [40] reported that of 334 TSTs placed in a cohort of asylum seekers, only 236 were read. In this study, when screened using TST, 5/236 (2%) of those read were positive. Of these five patients, three were started on treatment. It is unclear if the remaining patients were not offered or declined LTBI treatment, and it is unclear how many completed treatment.
Latent TB infection screening and treatment outcomes in health care workers
Five studies reported on LTBI screening in this cohort (Table 8) [29, 42,43,44, 46]. Two studies reported on the prevalence of LTBI in health care workers screened using a TST [29, 46]. In a cohort of new entrant health care workers, 32% had LTBI [29] while in health care workers with significant exposure to infectious TB, the prevalence was 56% [46]. Kelly et al. [42] reported that of new entrant health care workers from overseas were screened using a TST or an IGRA, 17% had a positive test result of which 85% were offered LTBI treatment [43]. Only 26% accepted treatment, all of whom completed treatment. Arya et al. [44] reported of 243 health care workers with a positive TST referred to a TB clinic, only 59% accepted LTBI treatment, but it is not reported how many were offered LTBI treatment. Of these, 62% completed treatment [44].
Discussion
This research presents a comprehensive review of studies describing LTBI prevalence and screening and treatment outcomes in Ireland and highlights the significant knowledge gaps. The findings demonstrate that there are few studies that are reliably informative as to the prevalence of LTBI across all risk cohorts in Ireland. Studies were all performed on a local or regional level. When considering only the studies where the risk of bias was moderate-low, the prevalence of a positive IGRA among immunosuppressed patients was 7% (IQR 5–7%). There is no published research describing the prevalence of LTBI in people from countries with a high incidence of TB, people who are homeless, people in prisons and people who use intravenous drugs in Ireland, and for asylum seekers, there were only two studies describing the prevalence of LTBI, both of which had a moderate or high risk of bias. Regarding health care workers, only two studies, both performed in the same centre and both with a high risk of bias, were informative as to the prevalence of LTBI. Despite these cohorts having an increased risk of TB in other low-incidence countries [47,48,49], it is unclear if the incidence of TB in these cohorts in Ireland is high because TB cases are not described according to these characteristics in recent national surveillance reports. However, a 2015 report describing risk factors for TB cases notified in 2013 reported that approximately 20 to 25% of cases had “high endemicity residence”, approximately 30% had “high endemicity origin” and approximately 10% had “substance abuse” [50]. Additionally, significant TB outbreaks have been reported in the Irish prison system within the previous decade [51]. Studies assessing the prevalence of LTBI and risk of TB reactivation in people from countries with a high incidence of TB, people who use drugs and prison inmates should be a future research priority in Ireland.
Research describing the cascade of LTBI care in Ireland was limited. Among immunosuppressed patients, treatment acceptance and completion appeared to be generally high, although the number of patients with LTBI described in these studies was small. Among TB case contacts, provider recommendation of treatment was reported as 61% in one study [31], treatment acceptance was reported as 31% and 67% [30][30], and treatment completion was reported as 33% and 77% [31, 35]. Among health care workers, two studies reported that the acceptance of LTBI treatment was generally low. There was insufficient information in the literature to describe the cascades of care in other cohorts and provide insight into where it should be improved. There were no studies which described the cost-effectiveness of LTBI screening and treatment, which are important if LTBI is to be managed programmatically at scale. A 2015 report describing risk factors for TB cases notified in 2013 reported that approximately 10%, 5% and 5% of TB cases occurred in TB case contacts, people with immunosuppressive illnesses and people on immunosuppressive medications, respectively [50]. Studies evaluating the cascade of LTBI care in PLWHIV should be prioritised, and further studies evaluating the cascade of LTBI care in patients on immunosuppressive treatments and TB case contacts should be encouraged. These studies would have utility when defining the diagnostic algorithm most appropriate for each target group in Ireland, which is key for effective programmatic management [4].
The strengths of this review are its rigorous methodology and that it is the first comprehensive review of TB research in Ireland, which establishes with certainty that scope and degree of research are needed. A weakness of this systematic review was that the research question, while was intentionally broad, could have been more explicitly defined at inception using the PICO model. With regard to abstract publications, the authors were not contacted to search for any further results. However, most abstracts included in this review described single-centre studies with a small sample size obtained using convenience sampling, limiting their utility when assessing the prevalence of LTBI. A limitation of this research was that there were few studies which were reliably informative as to the prevalence of LTBI because the risk of sampling bias was high across almost all studies. Therefore, the limited prevalence estimates reported in this review should be interpreted with caution.
Intensified research and innovation is a strategic pillar of the WHO End TB strategy, which should be adapted at a country level with global collaboration [2]. Studies meeting the identified research needs must be performed. The WHO describes the components of an enabling environment for high-quality research, which has relevance for LTBI research in Ireland [52, 53]. These components include having a national TB research network. This could enhance collaboration between researchers, health care providers and patients and coordinate local and national TB research activities to align with national TB programme priorities [52]. The WHO recommends the formation of a country-specific TB research agenda and strategic plan to guide country-specific actions [52]. Other low TB incidence countries have advanced national LTBI research in cohorts they have identified as at risk, such as in Canada and England, where LTBI research priorities have been outlined in TB elimination strategies [14, 54]. In Canada, the Public Health Agency have funded studies in Inuit people [55,56,57] and in the UK, Public Health England [8, 11], the National Institute of Health Research [8, 10, 11, 58]and the Medical Research Council [9, 10] have funded LTBI research in people from countries with a high incidence of TB. TB research networks must not only contribute to local and national TB elimination efforts but also global TB elimination efforts through international collaboration [2]. Other European countries such as the Netherlands are prime examples of how countries with a low incidence of TB can be global leaders in transnational collaboration for TB research by funding and developing in their institutions TB researchers and research programmes that are guided by a national TB research agenda and the WHO Global TB Research Agenda [59].
The WHO advises that an enabling environment for TB research should have sufficient local researchers with the necessary profiles in TB research and incentives to retain them in employment and that there should be specialized training on TB for new researchers [52]. Although there are many researchers involved in other aspects of TB in Ireland, such as host–pathogen response, drug development and TB diagnostics [60, 61], such is the scale of the identified LTBI epidemiological and cascade of care research needs that to meet them, dedicated TB research positions should be created within research institutions and form part of a TB-network. A high-quality research network with a well-defined research plan and strategy and the opportunity for international collaboration could attract new researchers to this field in Ireland and contribute to achieving TB elimination. The research needs identified in this systematic review would be best met by inclusion in a TB research agenda and strategic plan and delivered through a TB-network that develops local, national and international TB research.
Conclusion
This systematic review has described what published research there is on the epidemiology and cascade of LTBI care in Ireland and identified knowledge gaps. A strategy for addressing these knowledge gaps has been proposed.
Appendix 1. PRISMA checklist
Section/topic | No | Checklist item | Reported on page no |
|---|---|---|---|
TITLE | |||
Title | 1 | Identify the report as a systematic review, meta-analysis, or both | 1 |
ABSTRACT | |||
Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | 2 |
Introduction | |||
Rationale | 3 | Describe the rationale for the review in the context of what is already known | 3–4 |
Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS) | 4 |
Methods | |||
Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number | 5 |
Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale | 5 |
Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | 5 |
Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | Appendix 2 |
Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | Page 5 and Appendix 2 |
Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | Page 5 and Appendix 2 |
Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made | 5 |
Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | Page 5, Appendix 2 and 3 |
Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means) | 5 |
Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency | 5 |
Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies) | Appendix 3 |
Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified | No additional analyses |
Results | |||
Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | Figure 1 |
Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations | Page 6 and Table 3 |
Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12) | Page 8 and Table 4 |
Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot | Pages 10–14 |
Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency | Pages 10–14 |
Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15) | Appendix 3 |
Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]) | No additional analyses |
Discussion | |||
Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers) | Pages 14–15 |
Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias) | Pages 14–15 |
Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | Page 15 |
Funding | |||
Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review | Page 16 |
Appendix 2. Protocol for a systematic review of studies evaluating latent TB screening in the Republic of Ireland
Introduction
Latent tuberculosis infection (LTBI) is a state of a persistent immune response to stimulation by Mycobacterium tuberculosis antigens with no evidence of clinically manifest active tuberculosis (TB) [299]. It is estimated that 24.8% of the world’s population has LTBI [465]. In high-income low-incidence TB countries, most TB disease occurs due to the reactivation of latent TB and not ongoing disease transmission [586]. The World Health Organization’s (WHO) End TB Strategy states that the identification and management of LTBI in groups of people at high risk of reactivation is an essential part of TB elimination in low-incidence countries [18]. The End TB Strategy also suggests that epidemiological research should be conducted to determine the burden of LTBI in various geographical settings and risk groups and as a basis for nationally and locally tailored interventions, including integrated community-based approaches [18]. The Health Protection Surveillance Centre Guidelines for the Prevention and Control of TB 2010 guidelines outline which groups should be prioritized for LTBI screening in Ireland and offer guidance as to the diagnostic approach for certain target groups (Table 9) [151]. However, these guidelines do not discuss strategies for service delivery and programmatic monitoring and evaluation. In this systematic review, we aim to determine what evidence exists to describe the epidemiology of LTBI in the Republic of Ireland. Knowledge of regional LTBI epidemiology is crucial to improve the programmatic management of LTBI.
Aim
We aim to describe the epidemiology of LTBI in the Republic of Ireland including its prevalence, screening outcomes, and treatment outcomes.
Objectives
To determine:
-
1.
What the prevalence of LTBI is in the Republic of Ireland
-
2.
What proportion of patients are being offered treatment
-
3.
What proportion of patients are accepting treatment
-
4.
What proportion of patients are completing treatment
-
5.
What the cost of LTBI screening is in the Republic of Ireland
Methods
A systematic literature review will be performed and reported in adherence with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [463].
Included studies
We will include all studies describing patients who were screened for LTBI in the Republic of Ireland. Studies must be published in English. Conference abstracts will be included for review. All studies published from 01 Jan. 2000 to 31 Dec. 2020 will be included. Studies must use any one or a combination of chest X-ray (CXR), tuberculin skin tests (TST) or interferon-gamma release assay (IGRA) to screen for LTBI. Clinical audits randomized controlled trials, diagnostic accuracy studies, retrospective cohort reviews, and prospective cohort reviews will be included. We plan to exclude any studies where it was not possible to ascertain data on only patients screened in the Republic of Ireland.
Outcomes
The outcomes chosen are screening test used, the proportion of people screened out of target population, the prevalence of a positive screening test in the target population, proportion of those diagnosed with LTBI who are offered treatment, the proportion of those diagnosed with LTBI who started treatment for LTBI, the proportion of those diagnosed with LTBI who completed treatment and the cost of performing screening and/or treatment of LTBI cases identified.
Search methods
We will search MEDLINE (via OVID), Embase, Web of Science and Google Scholar. The references of the included studies will also be searched. All available published conference abstracts will be searched from the Irish Thoracic Society (published online in the Irish Journal of Medical Science), Irish Society of Rheumatology (published online in the Irish Journal of Medical Science), Irish Society of Gastroenterology (published online in the Irish Journal of Medical Science), Royal College of Physicians Ireland Faculty of Public Health and Faculty of Occupational Medicine (published online in the Irish Journal of Medical Science), the Infectious Diseases Society of Ireland (published online at www.idsi.ie) and the Irish Nephrology Society (www.nephrology.ie). A full list of conference abstracts is to be searched.
Search strategy
The search strategy, including a full list of the conference abstracts to be searched, is shown below. Both free-text terms and MeSH terms will be used in EMBASE, Medline and Web of Science. O’Connell J. designed the search strategy. The search will be performed independently by O’Connell J. and Gibbons C. All records returned will be screened by O’Connell J. and Gibbons C. independently. All records which are deemed to meet the study inclusion criteria will then have their full articles reviewed. All articles included for full-text review will have their references searched for other studies that meet inclusion criteria.
Embase search strategy.
1 | ‘tuberculosis’/exp OR ‘tuberculosis’ |
|---|---|
2 | ‘mycobacterium tuberculosis’/exp OR ‘mycobacterium tuberculosis’ |
3 | ‘latent tuberculosis’/exp OR ‘latent tuberculosis’ |
4 | #1 OR #2 OR #3 |
5 | ‘ireland’/exp OR ‘ireland’ |
6 | ‘screening’/exp OR ‘screening’ |
7 | ‘microorganism detection’/exp OR ‘microorganism detection’ |
8 | ‘assessment of humans’/exp OR ‘assessment of humans’ |
9 | #6 OR #7 OR #8 |
10 | (‘interferon’/exp OR interferon) AND gamma AND (‘release’/exp OR release) AND (‘assay’/exp OR assay) |
11 | ‘tuberculin test’/exp OR ‘tuberculin test’ |
12 | #10 OR #11 |
13 | #4 AND #5 AND #9 AND #12 |
MEDLINE (OVID) search strategy
-
1.
exp Mycobacterium tuberculosis/
-
2.
exp Tuberculosis/
-
3.
exp Latent Tuberculosis/
-
4.
1 or 2 or 3
-
5.
(“tuberculosis” or “mycobacterium tuberculosis” or “latent tuberculosis”).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms].
-
6.
exp Ireland/
-
7.
(ireland or Irish).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms].
-
8.
(screen* or test* or assess*).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms].
-
9.
exp Mass Screening/
-
10.
“interferon-gamma release assay”.mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms].
-
11.
exp Interferon-gamma Release Tests/
-
12.
exp Tuberculin Test/
-
13.
(“tuberculin” or “mantoux”).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms].
-
14.
4 or 5.
-
15.
6 or 7.
-
16.
8 or 9.
-
17.
10 or 11 or 12 or 13.
-
18.
14 and 15 and 16 and 17.
Web of Science search strategy
-
1.
TS = (Tuberculosis OR Latent Tuberculosis OR Mycobacterium Tuberculosis).
-
2.
TS = (Tuberculosis OR Latent NEAR/1 Tuberculosis OR Mycobacterium NEAR/1 Tuberculosis).
-
3.
TS = (Screen* OR Assess* OR Detect*).
-
4.
ALL = (‘Interferon Gamma Release Assay’ OR Mantoux OR Tuberculin).
-
5.
#2 OR #1 [ ALL TB]
-
6.
#4 OR #3 [ALL SCREEN OR TEST]
-
7.
#6 AND #5 [TB AND SCREEN].
-
8.
TS = (Ireland OR Irish).
-
9.
#8 AND #7 [TB IRELAND].
-
10.
ALL = (veterinary OR livestock OR agricultur* OR cattle OR sheep OR pigs OR chickens OR avian).
-
11.
#9 NOT #10 [REMOVE LIVESTOCK]
Google Scholar search strategy
[Tuberculosis OR TB OR Mycobacterium Tuberculosis] AND [Ireland OR Irish] AND [Screen* OR Detect* OR Assess* OR Test*] AND [Tuberculin OR Mantoux OR Interferon Gamma Release Assay].
Conference Abstract Search Strategy
The conference abstract publications identified for searching are shown in Table 10.
Risk of bias assessment
We will perform a risk of bias assessment using a tool designed for assessing the risk of bias in TB prevalence studies (Appendix 3), which was based on guidance from Cochrane and an existing risk of bias tool for prevalence studies [465]. The risk of bias assessment will be performed by O’Connell J. and Gibbons C. Any disagreements in the risk of bias assessment of studies included will be resolved by mutual agreement.
Data extraction
Data extraction will be performed by two reviewers, O’Connell J. and Li B. Both authors will extract the data independently into a data collection tool. Any disagreement in data extracted will be resolved by discussion and mutual agreement. Data points for extraction for shown in Table 11.
References
1. Rosales-Klintz S, Bruchfeld J, Haas W, Heldal E, Houben RM, van Kessel F, et al. Guidance for programmatic management of latent tuberculosis infection in the European Union/European Economic Area. Eur Respiratory Soc; 2019.
2. Cohen A, Mathiasen VD, Schӧn T, Wejse C. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. European Respiratory Journal. Eur Respiratory Soc; 2019;54(3).
3. France AM, Grant J, Kammerer JS, Navin TR. A field-validated approach using surveillance and genotyping data to estimate tuberculosis attributable to recent transmission in the United States. American journal of epidemiology. Oxford University Press; 2015;182(9):799–807.
4. Uplekar M, Weil D, Lonnroth K, Jaramillo E, Lienhardt C, Dias HM, et al. WHO’s new end TB strategy. The Lancet. Elsevier; 2015;385(9979):1799–801.
5. National Tuberculosis Advisory Committee, Guidelines on the Prevention and Control of Tuberculosis in Ireland 2010. Health Protection Surveillance Centre (HPSC); 2010.
6. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Journal of Clinical Epidemiology. 2009;62(10):1006–12.
Appendix 3. Risk of bias assessment tool
For the prevalence of a positive screening test, we assessed the risk of bias using a tool designed for TB prevalence studies that was derived from on an existing tool for prevalence studies (Table 12) [354]. The tool assesses the risk of bias across four domains, and each domain is scored on a scale of 0–2. A maximum score of 8 can be given for studies which score a low risk of bias across all domains. A minimum score of 0 can be given for studies which score a high risk of bias across all domains. The risk of bias assessment was performed independently by O’Connell J. and Gibbons C. Any disagreements in the risk of bias assessment of studies included were resolved by mutual agreement. The outcome of the risk of bias assessment is shown in full in Table 13.
The risk of bias relating to the other outcomes in the cascade of care was not formally assessed with a risk of bias tool because most of the items in risk of bias tools for non-randomized studies, such as the ROBINS-I tool [355] or the Newcastle Ottawa Scale [356], are not applicable to the primarily descriptive non-interventional studies that describe the cascade of TB care, and this limitation has always been encountered in other systematic reviews of TB cascades of care [291].
References
Houben RM, Dodd PJ (2016) The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS 13(10):e1002152
World Health Organisation (2015) End TB Strategy: Global strategy and targets for tuberculosis prevention, care and control after 2015 [Internet]. 2014 [cited 2021 Aug 5]. Available from: https://www.who.int/tb/post2015_strategy/en/
Lӧnnroth K, Migliori GB, Abubakar I et al (2015) Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respiratory Soc 45(4):928–52
Rosales-Klintz S, Bruchfeld J, Haas W et al (2019) Guidance for programmatic management of latent tuberculosis infection in the European Union/European Economic Area. Eur Respiratory Soc
Gilbert RL, Antoine D, French C et al (2009) The impact of immigration on tuberculosis rates in the United Kingdom compared with other European countries. Int J Tuberc Lung Dis 13(5):645–51
Aldridge RW, Zenner D, White PJ et al (2016) Tuberculosis in migrants moving from high-incidence to low-incidence countries: a population-based cohort study of 519 955 migrants screened before entry to England, Wales, and Northern Ireland. The Lancet. Elsevier 388(10059):2510–8
Choudhury IW, West CR, Ormerod LP et al (2013) The outcome of a cohort of tuberculin-positive predominantly South Asian new entrants aged 16–34 to the UK: Blackburn 1989–2001. J Public Health Med [Internet] 36(3):390–5. Available from: https://doi.org/10.1093/pubmed/fdt110
Zenner D, Loutet MG, Harris R et al (2017) Evaluating 17 years of latent tuberculosis infection screening in north-west England: a retrospective cohort study of reactivation. Eur Respiratory Soc 50(1)
Pareek M, Watson JP, Ormerod LP et al (2011) Screening of immigrants in the UK for imported latent tuberculosis: a multicentre cohort study and cost-effectiveness analysis. The Lancet infectious diseases. Elsevier 11(6):435–44
Pareek M, Bond M, Shorey J et al (2013) Community-based evaluation of immigrant tuberculosis screening using interferon gamma release assays and tuberculin skin testing: observational study and economic analysis. Thorax. BMJ Publishing Group Ltd 68(3):230–9
Loutet MG, Burman M, Jayasekera N et al (2018) National roll-out of latent tuberculosis testing and treatment for new migrants in England: a retrospective evaluation in a high-incidence area. Eur Respiratory Soc 51(1)
Walker C-L, Duffield K, Kaur H et al (2018) Acceptability of latent tuberculosis testing of migrants in a college environment in England. Public health Elsevier 158:55–60
National latent TB infection testing and treatment programme (2015). [cited 2021 Feb 14]; Available from: https://www.england.nhs.uk/tuberculosis-programme/area-for-action-8-national-latent-tb-infection-testing-and-treatment-programme/
Public Health England (2015) Collaborative Tuberculosis Strategy for England 2015–2020 [Internet]. [cited 2021 Aug 8]. Available from: https://www.gov.uk/government/publications/collaborative-tuberculosis-strategy-for-england
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. J Clin Epidemiol 62(10):1006–1012
Cohen A, Mathiasen VD, Schӧn T, Wejse C (2019) The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur Respiratory Soc 54(3)
Gnanasekaran R, Olupitan O, Silke C et al (2016) Adherence to anti tuberculosis screening protocols before starting a patient on biological therapy for inflammatory arthritis. Ir J Med Sci 185 (Suppl 5):187. Available from: https://doi.org/10.1007/s11845-016-1467-x
O’Flynn E (2007) Pre-biologic screening in autoimmune disease [Internet]. Ir J Med Sci [cited 2021 Aug 6]. p. 71. Available from: https://doi.org/10.1007/s11845-007-0112-0
Awan S, Bannon C, O’Sullivan M et al (2012) Can Quanti-FERON-TB replace TST (mantoux) as a screening tool prior to (biologics) anti-TNF therapy. Ir J Med Sci 181(2):49–81. Available from: https://doi.org/10.1007/s11845-012-0832-7
O’Flynn E (2014) Performance and benefits of replacing Mantoux test with QFT in screening for latent TB in patients prior to anti-TNF therapy, ISR Autumn 2013 Meeting. Ir J Med Sci [Internet] 183(3):87–118. Available from: https://doi.org/10.1007/s11845-014-1108-1
Hurley K, Farrell S, Keogh BA et al (2007) Screening for latent tuberculosis infection using gamma interferon and tuberculin skin tests prior to transplant or anti-TNF therapy, Irish Thoracic Society Annual Scientific Meeting. Ir J Med Sci [Internet] 176(10):385–426. Available from: https://doi.org/10.1007/s11845-007-0092-0
Safwat H (2015) Retrospective review of IGRA (interferon-gamma release assay) testing in Sligo General Hospital, Irish Thoracic Society Annual Scientific Meeting 2015. Ir J Med Sci (1971 -) [Internet] 184(11):475–547. Available from: https://doi.org/10.1007/s11845-015-1356-8
Haroon M, Martin U, Devlin J et al (2012) High incidence of intolerance to tuberculosis chemoprophylaxis. Rheumatol Int 32(1), 33-37. Available from: https://doi.org/10.1007/s00296-010-1571-6
O’Flynn E (2012) QFT testing in mantoux negative patients commencing anti-TNF therapy identifies additional at risk patients. Ir J Med Sci 181(2):49–81. Available from: https://doi.org/10.1007/s11845-012-0832-7
Martin J, Walsh C, Gibbs A et al (2010) Comparison of interferon γ release assays and conventional screening tests before tumour necrosis factor α blockade in patients with inflammatory arthritis. Ann Rheum Dis 69(01):181. Available from: http://ard.bmj.com/content/69/01/181.abstract
Jordan N, Kavanagh P, Dooley P, McCarthy C (2009) QFT Gold screening for latent tuberculosis- Cost comparison with mantoux testing [Internet]. Rheumatology. [cited 2021 Aug 6] i58–i71. Available from: https://doi.org/10.1093/rheumatology/kep723
Kelly A, Kirby B (2018) An audit of compliance with tuberculosis screening prior to treatment with biologics in psoriasis. Clin Exp Dermatol 43(5):611–611. Available from: https://doi.org/10.1111/ced.13413
Ni Cheallaigh C, Fitzgerald I, Grace J et al (2013) Interferon gamma release assays for the diagnosis of latent TB infection in HIV-infected individuals in a low TB burden country. PloS one 8(1):e53330
Ali S, Chew N, Manning P et al (2005) The prevalence of latent pulmonary tuberculosis (ltb) in a normal and a high risk population group. CHEST [Internet]128(4):397S. Available from: https://doi.org/10.1378/chest.128.4_MeetingAbstracts.397S-a
Higgins C, O’Donovan D, O’ Regan A et al (2016) An outbreak of tuberculosis on an Irish Island 2016 [Internet]. [cited 2021 Aug 6]. Available from: www.idsi.ie
Glynn H, O’Sullivan M (2014) An evaluation of the outcome of contact tracing of all sporadic cases of TB notified in cork over a 6-month period, Proceedings of the RAMI Intern Section Meeting, 18th January 2014 [Internet]. Ir J Med Sci (1971-2014) [cited 2021 Aug 6] 119–99. Available from: https://doi.org/10.1007/s11845-014-1153-9
O’Donovan D, Higgins C, Cloughley R et al (2014) Proceedings of the RAMI Intern Section Meeting, 18th January 2014. TB outbreak among students in higher education. Ir J Med Sci (1971) 183(4):119–99. Available from: https://doi.org/10.1007/s11845-014-1153-9
O’Meara M, Scully M, Keogh BA, Keelaghan A (2005) Contact tracing of tuberculosis in a school setting and the relevance of BCG in this population. Ir Med J 98(9):263–265 (PMID: 16300103)
O’Sullivan MB (2000) A Teenage tuberculosis cluster, Faculty of Public Health Medicine Summer Scientific Meeting. Ir J Med Sci [Internet] 169(4):54. Available from: https://doi.org/10.1007/BF03169093
Bambury N, Buckley C, MacSweeney M et al (2018) A review of management of latent tuberculosis infection (LTBI) in a TB contact tracing service in Cork, Ireland. Revue d’Épidémiologie et de Santé Publique [Internet] 66:S259–S260. Available from: http://www.sciencedirect.com/science/article/pii/S0398762018307661
Gaensbauer JT, Vandaleur M, O’Neil M et al (2009) BCG protects toddlers during a tuberculosis outbreak. Archives of disease in childhood. BMJ Publishing Group Ltd 94(5):392–3
Hennessy B (2008) Is tuberculin testing before BCG vaccination necessary for children over three months of age? Ir Med J 101(3):72–74
Iroh Tam PY, Menon A, Butler K (2010) A review of tuberculosis-related referrals among children in Ireland. Ir J Med Sci 179(2):251–254
Millar N, Ryan FM, Burke M et al (2006) Infectious Disease News; Vol. 4 (4), December 2006. Health Service Executive (HSE)South (Cork & Kerry), Department of Public Health. Available from: http://hdl.handle.net/10147/65858
Doyle SM (2006) An evaluation and audit of the asylum seeker communicable disease screening service in the Eastern region: a report submitted for membership of the Faculty of Public Health Medicine of the Royal College of Physicians of Ireland. Available from: http://hdl.handle.net/10147/263872
Smyth R, Nadarajan P, Gileece A, Cormican L (2012) Screening healthcare workers for Mycobacterium TB: Is QFT-G now the test of choice? Eur Respiratory Soc.
Kelly S, Reid A, Noone P et al (2018) 1480 A description of the effectiveness of screening overseas workers for latent tb. J Occup Med [Internet] 75(Suppl 2):A167. Available from: http://oem.bmj.com/content/75/Suppl_2/A167.3.abstract
Power S, Sim J, Gallagher J, Greiner B (2010) A study to compare chest X-ray reports on overseas nursing recruits. Irish medical journal [Internet]. Available from: http://hdl.handle.net/10147/125207
Arya A, Thornhill J, Noonan N, Keane J, (2007) Irish Thoracic Society Annual Scientific Meeting 9th and 10th November 2007 [Internet] [cited 2021 Aug 6]. Available from: https://doi.org/10.1007/s11845-007-0092-0.pdf
O’Flynn E (2012) QFT testing in mantoux negative patients commencing anti-TNF therapy identifies additional at risk patients. Ir J Med Sci 181:S58
Corr A, Hurley K, Dunican E et al (2009) Factors influencing acceptance of latent tuberculosis infection treatment in healthcare workers [Internet]. Ir J Med Sci [cited 2021 Aug 6]. Available from: https://link.springer.com/journal/11845/volumes-and-issues/178-11/supplement
Story A, Murad S, Roberts W et al (2007) Tuberculosis in London: the importance of homelessness, problem drug use and prison. Thorax. BMJ Publishing Group Ltd 62(8):667–71
Pareek M, Greenaway C, Noori T et al (2016) The impact of migration on tuberculosis epidemiology and control in high-income countries: a review. BMC medicine. Springer 14(1):48.
Peters C, Kozak A, Nienhaus A, Schablon A (2020) Risk of occupational latent tuberculosis infection among health personnel measured by interferon-gamma release assays in low incidence countries—a systematic review and meta-analysis. Int J Environ Research Public Health. Multidisciplinary Digital Publishing Institute 17(2):581
Tuberculosis Subcommittee (2013) Tuberculosis Control Review. Health Service Executive, April, 2015
Keegan N, Lyons J, McDonnell C et al (2012) A prisoner of TB—a case series of infection [Abstract] Irish Thoracic Society Annual Scientific Meeting. Ir J Med Sci 181:369–437
World Health Organization (2021) Situational assessment checklist to guide implementation of the global strategy for tuberculosis research and innovation, 2021
World Health Organization (2015) Implementing the end TB strategy: the essentials [Internet]. [cited 2021 Aug 6]. Available from: https://www.who.int/tb/publications/2015/end_tb_essential.
Kanatami IT (2018) Inuit Tuberculosis Elimination Framework [Internet]. Inuit Tapiriit Kanatami. [cited 2021 Aug 6]. Available from: https://www.itk.ca/inuittbeliminationframework/#:~:text=%E2%80%9CEliminating%20TB%20among%20Inuit%20in,%2C%20communities%20and%20healthcare%20professionals.%E2%80%9D
Alvarez G, Van Dyk D, Mallick R et al (2020) The implementation of rifapentine and isoniazid (3HP) in two remote Arctic communities with a predominantly Inuit population, the Taima TB 3HP study. International journal of circumpolar health. Taylor Francis 79(1):1758501
Alvarez G, Van Dyk D, Davies N et al (2014) The feasibility of the interferon gamma release assay and predictors of discordance with the tuberculin skin test for the diagnosis of latent tuberculosis infection in a remote aboriginal community. PLoS One 9(11):e111986
Pease C, Zwerling A, Mallick R et al (2019) The latent tuberculosis infection cascade of care in Iqaluit, Nunavut, 2012–2016. BMC infectious diseases. Springer 19(1):890
Berrocal-Almanza LC, Harris R, Lalor MK et al (2019) Effectiveness of pre-entry active tuberculosis and post-entry latent tuberculosis screening in new entrants to the UK: a retrospective, population-based cohort study. The Lancet Infectious Diseases. Elsevier 19(11):1191–201.
KNCV Tuberculosis Foundation (2015) Tuberculosis Research in the Netherlands, Innovate to Accelerate Global Tuberculosis Elimination [Internet]. [cited 2021 Jul 10]. Available from: https://www.kncvtbc.org/uploaded/2015/10/TBC1538_Whitepaper_WEB.pdf
Professor Joseph Keane, Research Summary, Trinity College Dublin (2019) [Internet]. [cited 2021 Jul 10]. Available from: https://www.tcd.ie/medicine/research/researchers/joe-keane.php
Royal College of Surgeons in Ireland (2019) Researchers develop new treatment for tuberculosis. [Internet] [cited 2021 Jul 17]. Available from: https://www.rcsi.com/impact/details/2019/04/researchers-develop-new-treatment-for-tuberculosis
Acknowledgements
The authors would like to acknowledge the assistance of Dr. Claire Gibbon and Mr. Brian Li in performing this systematic review.
Funding
Open Access funding provided by the IReL Consortium. Salary of the first author was funded by the Royal College of Surgeons in Ireland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
O’Connell, J., de Barra, E. & McConkey, S. Systematic review of latent tuberculosis infection research to inform programmatic management in Ireland. Ir J Med Sci 191, 1485–1504 (2022). https://doi.org/10.1007/s11845-021-02779-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-021-02779-w