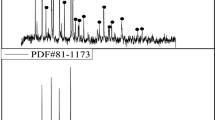
Abstract
A novel method for the recovery of Li from cathode material of waste ternary lithium-ion batteries is proposed, wherein water-based leaching of the reduction roasting product was conducted. First, the effects of leaching temperature, leaching time, liquid–solid ratio, and CO2 aeration rate on the recovery of Li were investigated, demonstrating that the leaching rate of Li can reach 79.95% under optimal conditions, i.e., a CO2 aeration rate of 200 mL/min, leaching time of 1.5 h, and liquid–solid ratio of 15:1, at 15°C. The leaching time and CO2 aeration rate had little impact, while the liquid–solid ratio and temperature were found to significantly affect the leaching rate of Li. Then, counter-current leaching experiments were carried out, and the total leaching rate of Li exceeded 98% after four stages with a liquid-to-solid ratio of 15:1. Battery-grade purity of the lithium carbonate product was obtained by evaporative crystallization.








Similar content being viewed by others
References
N.A. Owen, O.R. Inderwildi, and D.A. King, Energy Policy 38, 4743 (2010).
B. Dunn, H. Kamath, and J.M. Tarascon, Science 334, 6058 (2011).
D. Deng, Energy. Sci. Eng. 3, 385 (2015).
A. Christian, and J. Gerfried, Energies 13, 6345 (2020).
B. Huang, Z. Pan, and X. Su, J. Power. Sources. 399, 274 (2018).
W.G. Lv, Z.H. Wang, H.B. Cao, Y. Sun, Y. Zhang, and Z. Sun, ACS Sustainable Chem. Eng. 6, 1504 (2018).
Y.F. Song, and Z.W. Zhao, Sep. Purif. Technol. 206, 335 (2018).
Y.Q. Wang, N. An, L. Wen, L. Wang, X.T. Jiang, F. Hou, Y.X. Yin, and J. Liang, J ENERGY CHEM. 55, 391 (2018).
M. Contestabile, S. Panero, and B. Scrosati, Sources 92, 65 (2001).
S. Natarajan, and V. Aravindan, Adv. Energy. Mater. 8, 1802303 (2018).
S.M. Shin, N.H. Kim, J.S. Sohn, D.H. Yang, and Y.H. Kim, Hydrometallurgy 79, 172 (2005).
C. Hanisch, W. Haselrieder, and A. Kwade, Glocalized Sol. Sustain. Manuf. https://doi.org/10.1007/978-3-642-19692-8_15 (2011).
D. Song, X. Wang, E. Zhou, P. Guo, and L. Zhang, J. Power. Sources. 232, 348 (2013).
T.C. Nirmale, B.B. Kale, and A.J. Varma, Int J Biol Macromol. 103, 1032 (2017).
J.H. Li, X.H. Li, Q.Y. Hu, Z.X. Wang, and J.C. Zhang, Hydrometallurgy 99, 7 (2010).
G.G. Wang, H.N. Zhang, T. Wu, B.R. Liu, Q. Huang, and Y.F. Su, Prog. Chem. 32, 2064 (2020).
X.P. Fan, C.H. Song, X.F. Lu, Y. Shi, S.L. Yang, F.H. Zheng, Y.G. Huang, K. Liu, H.Q. Wang, and Q.Y. Li, J. Alloy. Compd. 863, 15 (2021).
L. Yang, G.X. Xi, and Y.B. Xi, Int. 41, 11498 (2015).
P.Y. Huo, L.Q. Zhang, and E.L. Zhou, J. Power. Sources. 232, 348 (2013).
R. Sattar, S. Ilyas, H.N. Bhatti, and A. Ghaffar, Sep. Purif. Technol. 209, 725 (2019).
S.P. Barik, G. Prabaharan, and L. Kumar, J. Clean. Prod. 147, 37 (2017).
W.F. Gao, J.L. Song, and H.B. Cao, J. Clean. Prod. 178, 833 (2018).
C.L. Zhang, B.X. Wang, and Z.Y. Li, Nonferrous Metals Eng. 10, 73 (2020).
M.C. Sun, H. Ye, and W.J. Chen, Nonferrous Metals (Extractive Metallurgy). 3, 68 (2019).
J. Nan, D. Han, and X. Zuo, J. Power. Sources. 152, 278 (2005).
G.S. Zeng, X.R. Deng, S.L. Luo, X.B. Luo, and J.P. Zou, J. Hazard. Mater. 199, 164 (2012).
S. Virolainen, M.F. Fini, A. Laitinen, and T. Sainio, Sep. Purif. Technol. 179, 274 (2017).
F. Vasilyev, S. Virolainen, and T. Sainio, Sep. Purif. Technol. 210, 530 (2019).
Y. Yang, S.L. Song, F. Jiang, J.H. Zhou, and W. Sun, J. Cleaner Prod. 186, 123 (2018).
L. Li, Y.F. Bian, X.X. Zhang, Y.B. Guan, E. Fan, F. Wu, and R.J. Chen, Waste Manage. 71, 362 (2018).
A.A. Thomas, E. Giuseppe, H. Robert, A. Pietro, and P. Francesca, J. Energy Chem. 35, 220 (2019).
L. Li, Y.F. Bian, X.X. Zhang, Y. Yao, Q. Xue, E. Fan, F. Wu, and R.J. Chen, Waste Manage. 85, 437 (2019).
Q. Meng, Y. Zhang, P. Dong, and F. Liang, J. Ind. Eng. Chem. 61, 133 (2018).
G.X. Ren, B. Pan, M.Q. Xie, J. Chen, F.G. Wang, and S.W. Xiao, Min. Metall. Eng. 35, 75 (2015).
X.H. Zhang, Y.B. Xie, H.B. Cao, F.H. Nawaz, and Y. Zhang, Waste Manage. 34, 1715 (2014).
C. Peng, F.P. Liu, Z.L. Wang, B.P. Wilson, and M. Lundstrom, J. Power. Sources. 415, 179 (2019).
Y. Yu, D. Wang, H. Chen, X. Zhang, and L. Yang, Chem. Res. Chin. Univ. 5, 908 (2020).
K. Yan, Z.Y. Xiong, Z.L. Liu, Z.F. Xu, R.X. Wang, and H.P. Nie, J. Central South Univ. Sci. Technol. 51, 3367 (2020).
J.A. Dean, Lange’s Handbook of Chemistry (Science Press, Beijing, 1991).
Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2019YFC1908404, No. 2019YFC1908405), the Natural Science Foundation of China (No. 51904124, No. 52064018, No. 51974140, No. 52004111), Key Projects of Jiangxi Key R&D Plan (No. 20192ACB70017, No. 20212ACB204015), the Distinguished Professor Program of Jinggang Scholars, China Institutions of Higher learning Jiangxi Province, the Program of Qingjiang Excellent Young Talents, Jiangxi University of Science and Technology (JXUSTQJYX2020012), Jiangxi Provincial Cultivation Program for Academic and Technical Leaders of Major Subjects (20212BCJL23052, 20212BCJ23006, 20212BCJ23007), the Natural Science Foundation of Jiangxi Province(20202BAB214015), China and Post-subsidy project of young talents plan of Ganzhou Science and Technology Bureau (No. 2020-14).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yan, K., Chen, Q., Xiong, Z. et al. A Novel Method for the Recovery of Li from Spent Lithium-Ion Batteries Using Reduction Roasting–Countercurrent Leaching. JOM 74, 3821–3832 (2022). https://doi.org/10.1007/s11837-022-05432-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-022-05432-8