Abstract
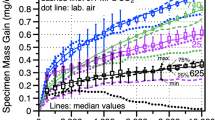
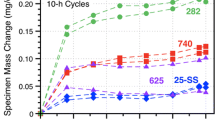
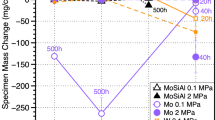
To understand and model performance in supercritical CO2 (sCO2) for high-efficiency, concentrating solar power (CSP) and fossil energy power cycles, reaction rates are compared at 750°C in 0.1 MPa CO2 and 30 MPa sCO2 as well as laboratory air as a baseline on structural materials such as Ni-based alloy 625. Due to the thin reaction products formed even after 5000 h, scanning transmission electron microscopy was used to study the Cr-rich surface oxide scale. The scales formed in CO2 and sCO2 had a much finer grain size with more voids observed in CO2. However, the observations on alloy 625 were complicated by Mo and Nb-rich precipitates in the adjacent substrate and Al internal oxidation. To simplify the system, a binary Ni-22Cr alloy was exposed for 1000 h in similar environments. After exposure in sCO2, there was an indication of carbon segregation detected on the Cr2O3 grain boundaries. After exposure in air, metallic Ni precipitates were observed in the scale that were not observed in the scale formed on alloy 625. The scale formed in air on a second Ni-22Cr model alloy with Mn and Si additions did not contain Ni precipitates, suggesting caution when drawing conclusions from model alloys.










Similar content being viewed by others
References
V. Dostal, P. Hejzlar, and M.J. Driscoll, Nucl. Technol. 154, 265 (2006).
B.D. Iverson, T.M. Conboy, J.J. Pasch, and A.M. Kruizenga, Appl. Energy 111, 957 (2013).
R.J. Allam, M.R. Palmer, G.W. Brown Jr, J. Fetvedt, D. Freed, H. Nomoto, M. Itoh, N. Okita, and C. Jones Jr, Energy Procedia 37, 1135 (2013).
V.T. Cheang, R.A. Hedderwick, and C. McGregor, Sol. Energy 113, 199 (2015).
D.J. Young, High Temperature Oxidation and Corrosion of Metals, 2nd ed. (Oxford: Elsevier, 2016).
D. Huenert and A. Kranzmann, NACE Paper 08-447, Houston, TX, presented at NACE Corrosion 2008, (New Orleans, LA, 2008)
B.A. Pint and J.R. Keiser, JOM 67, 2615 (2015).
B.A. Pint, R.G. Brese, and J.R. Keiser, Mater. Corros. 68, 151 (2017).
B.A. Pint, R. Brese, and J.R. Keiser, ASME Paper #GT2017-65066, for the International Gas Turbine & Aeroengine Congress & Exhibition (Charlotte, NC, 2017)
B.A. Pint, K.A. Unocic, R.G. Brese, and J.R. Keiser, Mater. High Temp. 35, 39 (2018).
E.G. Feher, Energy Convers. 8, 85 (1968).
H.E. McCoy, Corrosion 21, 84 (1965).
W.R. Martin and J.R. Weir, J. Nucl. Mater. 16, 19 (1965).
H.E. Evans, D.A. Hilton, and R.A. Holm, Oxid. Met. 10, 149 (1976).
J.C.P. Garrett, J.T. Crook, S.K. Lister, P.J. Nolan, and J.A. Twelves, Corros. Sci. 22, 37 (1982).
P.C. Rowlands, J.C.P. Garrett, L.A. Popple, A. Whittaker, and A. Hoaskey, Nucl. Energy 25, 267 (1986).
Y. Gong, D.J. Young, P. Kontis, Y.L. Chiu, H. Larsson, A. Shin, J.M. Pearson, M.P. Moody, and R.C. Reed, Acta Mater. 130, 361 (2017).
C.T. Fujii and R.A. Meussner, J. Electrochem. Soc. 114, 435 (1967).
F. Rouillard, F. Charton, and G. Moine, Corrosion 67, 095001 (2011).
T. Furukawa and F. Rouillard, Prog. Nucl. Energy 82, 136 (2015).
R.I. Olivares, D.J. Young, P. Marvig, and W. Stein, Oxid. Met. 84, 585 (2015).
R. Viswanathan, J.F. Henry, J. Tanzosh, G. Stanko, J. Shingledecker, B. Vitalis, and R. Purgert, J. Mater. Eng. Perf. 14, 281 (2005).
G.C. Wood, I.G. Wright, T. Hodgkiess, and D.P. Whittle, Werk. Korr. 21, 900 (1970).
G.H. Meier, W.C. Coons, and R.A. Perkins, Oxid. Met. 17, 235 (1982).
B. Jönsson and C. Svedberg, Mater. Sci. Forum 251–254, 551 (1997).
I.G. Wright and R.B. Dooley, Int. Mater. Rev. 55, 129 (2010).
N. Mu, K.Y. Jung, N.M. Yanar, G.H. Meier, F.S. Pettit, and G.R. Holcomb, Oxid. Met. 78, 221 (2012).
E. Essuman, L.R. Walker, P.J. Maziasz, and B.A. Pint, Mater. Sci. Technol. 29, 822 (2013).
C.H. Oh, T. Lillo, W. Windes, T. Totemeier, B. Ward, R. Moore, and R. Barner, Idaho National Laboratory Report INL/EXT-06-01271, (2006)
H.J. Lee, H. Kim, S.H. Kim, and C. Jang, Corros. Sci. 99, 227 (2015).
J. Mahaffey, D. Adam, A. Brittan, M. Anderson, and K. Sridharan, Oxid. Met. 86, 567 (2016).
L.M. Pike, in Superalloys 2008, eds. by R.C. Reed et al. TMS (Warrendale, PA, 2008), p. 191)
A. Chyrkin, P. Huczkowski, V. Shemet, L. Singheiser, and W.J. Quadakkers, Oxid. Met. 75, 143 (2011).
M.J. Lance and B.A. Pint, in Proceedings of the 6th International Symposium on Supercritical CO 2 Power Cycles (Pittsburgh, PA, 2018), Paper #117 (2018)
B.A. Pint and K.A. Unocic, Oxid. Met. 87, 515 (2017).
Z. Zeng, K. Natesan, Z. Cai, and S.B. Darling, Nat. Mater. 7, 641 (2008).
D.J. Young, T.D. Nguyen, P. Felfer, J. Zhang, and J.M. Cairney, Scripta Mater. 77, 29 (2014).
I. Wolf and H.J. Grabke, Solid State Commun. 54, 5 (1985).
T.D. Nguyen, J. Zhang, and D.J. Young, Corr. Sci. 76, 231 (2013).
W.J. Quadakkers, H. Holzbrecher, K.G. Briefs, and H. Beske, Oxid. Met. 32, 67 (1989).
Acknowledgements
The author would like to thank M. Howell, M. Stephens, G. Garner, T. Lowe, T. Jordan, D. Coffey and C. Parrish at ORNL for assistance with the experimental work and S. Dryepondt and L. Allard for comments on the manuscript. The authors appreciate the donation of alloy 625 from Haynes International. This research was funded by the US Department of Energy’s Office of Energy Efficiency and Renewable Energy, Solar Energy Technology Office: SuNLaMP award number DE-EE0001556 and by the Office of Fossil Energy (Grant No. FEAA123), Crosscutting Technology Program. The STEM work was supported by the Office of Nuclear Energy, Fuel Cycle R&D Program and the Nuclear Science User Facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pint, B.A., Unocic, K.A. The Effect of CO2 Pressure on Chromia Scale Microstructure at 750°C. JOM 70, 1511–1519 (2018). https://doi.org/10.1007/s11837-018-2963-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-018-2963-4