Abstract
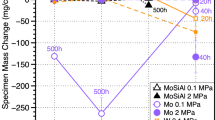
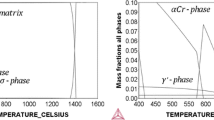
The oxidation behavior of several Fe and Ni commercial alloys was evaluated in high-temperature environments expected in next-generation supercritical carbon dioxide power cycles. The alloys were exposed to pure CO2 at 0.1 and 20 MPa and to CO2 containing H2O, O2, with and without SO2 at 0.1 MPa, at temperatures of 550°C (Fe alloys) and 700–750°C (Ni alloys). For comparison, the alloys were also exposed to air and supercritical steam. Low-Cr ferritic Fe alloys formed Fe-rich oxides leading to high oxidation rates in pure CO2, while high-Cr austenitic Fe alloys formed Cr-rich oxides leading to low oxidation rates. H2O and O2 impurities had negligible effect on the oxidation behavior of all Fe alloys. The addition of SO2 had negligible effect on the oxidation of ferritic Fe alloys, but significantly enhanced the rate for austenitic Fe alloys, where S in the oxide and underlying alloy contributed to the transition from Cr-rich to Fe-rich oxide growth. Ni alloys formed Cr-rich oxides and showed low oxidation rates in all CO2-containing environments which were very similar to those obtained in air and supercritical steam. Little or no effect of pressure was observed for any of the alloys exposed to pure CO2.





Similar content being viewed by others
References
D. Thimsen, R.A. Dennis, K. Brun, B.A. Pint, in Fundamentals and Applications of Supercritical Carbon Dioxide (sCO 2 ) Based Power Cycles, ed. K. Brun, P. Friedman, R.A. Dennis (Woodhead Publishing, Sawston, 2017), p. 415.
H. McCoy, Corrosion 21, 84 (1965).
W. Martin and J. Weir, J. Nucl. Mater. 16, 19 (1965).
R. Olivares, D. Young, T. Nguyen, and P. Marvig, Oxid. Met. (2018). https://doi.org/10.1007/s11085-017-9820-7.
B. Pint, K. Unocic, R. Brese, and J. Keiser, Mater. High Temp. 35, 39 (2017).
R.P. Oleksak, C.S. Carney, G.R. Holcomb, and Ö.N. Doğan, Oxid. Met. (2017). https://doi.org/10.1007/s11085-017-9821-6.
R.G. Brese, J.R. Keiser, and B.A. Pint, Oxid. Met. 87, 631 (2017).
F. Rouillard and T. Furukawa, Corros. Sci. 105, 120 (2016).
J. Mahaffey, D. Adam, A. Brittan, M. Anderson, and K. Sridharan, Oxid. Met. 86, 567 (2016).
H.J. Lee, G.O. Subramanian, S.H. Kim, and C. Jang, Corros. Sci. 111, 649 (2016).
G.R. Holcomb, C. Carney, and Ö.N. Doğan, Corros. Sci. 109, 22 (2016).
R. Olivares, D. Young, P. Marvig, and W. Stein, Oxid. Met. 84, 585 (2015).
H.J. Lee, H. Kim, S.H. Kim, and C. Jang, Corros. Sci. 99, 227 (2015).
T. Furukawa and F. Rouillard, Prog. Nucl. Energy 82, 136 (2015).
L.-F. He, P. Roman, B. Leng, K. Sridharan, M. Anderson, and T.R. Allen, Corros. Sci. 82, 67 (2014).
V. Firouzdor, K. Sridharan, G. Cao, M. Anderson, and T.R. Allen, Corros. Sci. 69, 281 (2013).
F. Rouillard, G. Moine, M. Tabarant, and J.C. Ruiz, Oxid. Met. 77, 57 (2012).
G. Cao, V. Firouzdor, K. Sridharan, M. Anderson, and T.R. Allen, Corros. Sci. 60, 246 (2012).
L. Tan, M. Anderson, D. Taylor, and T.R. Allen, Corros. Sci. 53, 3273 (2011).
T. Furukawa, Y. Inagaki, and M. Aritomi, Prog. Nucl. Energy 53, 1050 (2011).
R. Allam, S. Martin, B. Forrest, J. Fetvedt, X. Lu, D. Freed, G.W. Brown, T. Sasaki, M. Itoh, and J. Manning, Energy Procedia 114, 5948 (2017).
B.A. Pint, R.G. Brese, and J.R. Keiser, Mater. Corros. 68, 151 (2017).
F. Rouillard, G. Moine, L. Martinelli, and J.C. Ruiz, Oxid. Met. 77, 27 (2012).
G.H. Meier, K. Jung, N. Mu, N.M. Yanar, F.S. Pettit, J. Pirón Abellán, T. Olszewski, L. Nieto Hierro, and W.J. Quadakkers, Oxid. Met. 74, 319 (2010).
H. McCoy and B. McNabb, ORNL/TM 5781 (Oak Ridge, TN: Oak Ridge National Laboratory, 1977).
R. Viswanathan, J. Shingledecker, and R. Purgert, Power 154, 41 (2010).
G.R. Holcomb, Oxid. Met. 82, 271 (2014).
W. Ruther, R. Schlueter, R. Lee, and R. Hart, Corrosion 22, 147 (1966).
J. Griess and W. Maxwell, ORNL-5771 (Oak Ridge, TN: Oak Ridge National Laboratory, 1981).
I. Wright, P. Tortorelli, and M. Schütze, EPRI Rep 1013666, 2007.
R.P. Oleksak, J.P. Baltrus, J. Nakano, A. Nakano, G.R. Holcomb, and Ö.N. Doğan, Corros. Sci. 125, 77 (2017).
Acknowledgements
This work was performed in support of the U.S. Department of Energy’s Fossil Energy Crosscutting Technology Research and Advanced Turbine Programs. The Research was executed through NETL Research and Innovation Center’s Advanced Alloy Development Field Work Proposal. Research performed by AECOM Staff was conducted under the RES Contract DE-FE0004000. We thank Christopher McKaig (NETL) for the preparation of samples for cross-sectional SEM analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
This project was funded by the Department of Energy, National Energy Technology Laboratory, an agency of the United States Government, through a support contract with AECOM. Neither the United States Government nor any agency thereof, nor any of their employees, nor AECOM, nor any of their employees, makes any warranty, expressed or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oleksak, R.P., Tylczak, J.H., Carney, C.S. et al. High-Temperature Oxidation of Commercial Alloys in Supercritical CO2 and Related Power Cycle Environments. JOM 70, 1527–1534 (2018). https://doi.org/10.1007/s11837-018-2952-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-018-2952-7