Abstract
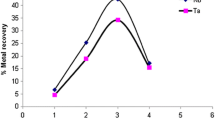
The similarity between Ta and Nb chemistry makes it difficult to find the appropriate reagents and chemical reactions for the separation of the two elements. This study investigated the precipitation behavior of TaF5 and NbF5 with p-phenylenediamine (PPDA). PPDA preferentially precipitated Nb from a 1:1 ratio of NbF5 and TaF5. Niobium recoveries of >80%, and only 4% Ta, were found in the precipitate of the reaction between (Nb/Ta)F5 and PPDA in ethanol. A separation factor of 100(9) indicated the potential for successful separation of Nb and Ta in a fluoride environment. A spectrophotometric study of the formation ratio of the newly formed Nb compound indicated a 1:1 metal:ligand ratio.







Similar content being viewed by others
References
A. Angulyansky, The Chemistry of Tantalum and Niobium Fluoride Compounds (Amsterdam: Elsevier B.V, 2004).
D.R. Sadoway and S.N. Flengas, Metall. Trans. B 11B, 57 (1980).
M. Nete, W. Purcell, and J.T. Nel, J. Fluor. Chem. 165, 20 (2014).
J.W. Mellor, A Comprehensive Treatise on Inorganic and Theoretical Chemistry, Longmans (London: Green & Co. LTD, 1947).
J.R. Werning and K.B. Higbie, Ind. Eng. Chem. 46, 2491 (1954).
L.P. Varga, W.D. Wakley, L.S. Nicolson, M.L. Madden, and J. Patterson, Anal. Chem. 37, 1003 (1965).
W. Kock and P. Paschen, JOM 41, 33 (1989).
X. Wang, S. Zheng, H. Xu, and Y. Zhang, Hydrometallurgy 98, 219 (2009).
H.H. Htwe and K.T. Lwin, World Acad. Sci. Eng. Technol. 46, 133 (2008).
O.N. Grebneva, I.V. Kubrakova, T.F. Kudinova, and N.M. Kuzmin, Spectrochem. Acta B 52, 1151 (1997).
G.E.M. Hall and J.C. Pelchat, J. Anal. Atom. Spectrom. 5, 339 (1990).
M. Nete, W. Purcell, and J.T. Nel, Hydrometallurgy 149, 31 (2014).
M.J. Kabangu and P.L. Crouse, Hydrometallurgy 129–130, 151 (2012).
M.J. Ungerer, D.J. van der Westhuizen, G. Lachmann, and H.M. Krieg, Hydrometallurgy 144–145, 195 (2014).
F.A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry: A Comprehensive Text, 2nd ed. (New York: Wiley, 1968).
W. He, F. Du, Y. Wu, Y. Wang, X. Liu, H. Liu, and X. Zhao, J. Fluor. Chem. 127, 809 (2006).
D. Deng, P. Deng, X. Wang, and X. Hou, Spectrosc. Lett. 42, 334 (2009).
D.A. Skoog, J.F. Holler, D.M. West, and S.R. Crouch, Fundamentals of Analytical Chemistry, 8th ed. (Australia: Thomson Brooks/Cole, 2004).
K. Nakamoto, Infrared Spectra of Inorganic and Coordination Compounds, 2nd ed. (New York: Wiley, 1970).
V. Sunitha and B. Muralidhara Reddy, Int. J. Curr. Res. Acad. Rev. 2, 159 (2014).
W. Levason, G. Reid, and W. Zhang, J. Fluorine Chem. 172, 62 (2015).
S.L. Benjamin, A. Hyslop, W. Levason, and G. Reid, J. Fluorine Chem. 137, 77 (2012).
W. Levason, M.E. Light, G. Reid, and W. Zhang, Dalton Trans. 43, 9557 (2014).
F. Marchetti, C. Pinzino, S. Zacchini, and G. Pampaloni, Angew. Chem. Int. 49, 5268 (2010).
J.W. Sibert, United States Patent US 6441164 B2 (Aug 27) (2002).
R. Gross and W. Kaim, Inorg. Chem. 26, 3596 (1987).
Acknowledgements
The authors thank the Research Fund of the University of the Free State, National Research Foundation of South Africa, Necsa and New Metals Development Network of the Advanced Metals Initiative of the Department of Science and Technology of South Africa for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nete, M., Purcell, W. & Nel, J.T. Separation of Niobium and Tantalum Pentafluoride by Selective Precipitation Using p-Phenylenediamine. JOM 68, 2817–2823 (2016). https://doi.org/10.1007/s11837-016-2003-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-016-2003-1