Abstract
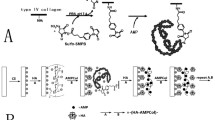
Implant-associated infections can have severe effects on the longevity of implant devices and they also represent a major cause of implant failures. Treating these infections associated with implants by antibiotics is not always an effective strategy due to poor penetration rates of antibiotics into biofilms. Additionally, emerging antibiotic resistance poses serious concerns. There is an urge to develop effective antibacterial surfaces that prevent bacterial adhesion and proliferation. A novel class of bacterial therapeutic agents, known as antimicrobial peptides (AMPs), are receiving increasing attention as an unconventional option to treat septic infection, partly due to their capacity to stimulate innate immune responses and for the difficulty of microorganisms to develop resistance towards them. While host and bacterial cells compete in determining the ultimate fate of the implant, functionalization of implant surfaces with AMPs can shift the balance and prevent implant infections. In the present study, we developed a novel chimeric peptide to functionalize the implant material surface. The chimeric peptide simultaneously presents two functionalities, with one domain binding to a titanium alloy implant surface through a titanium-binding domain while the other domain displays an antimicrobial property. This approach gains strength through control over the bio-material interfaces, a property built upon molecular recognition and self-assembly through a titanium alloy binding domain in the chimeric peptide. The efficiency of chimeric peptide both in-solution and absorbed onto titanium alloy surface was evaluated in vitro against three common human host infectious bacteria, Streptococcus mutans, Staphylococcus epidermidis, and Escherichia coli. In biological interactions such as occur on implants, it is the surface and the interface that dictate the ultimate outcome. Controlling the implant surface by creating an interface composed chimeric peptides may therefore open up new possibilities to modify the implant site and tailor it to a desirable bioactivity.







Similar content being viewed by others
References
S. Bauer, P. Schmuki, K. von der Mark, and J. Park, Prog. Mater. Sci. 58, 261 (2013).
D. Puleo and A. Nanci, Biomaterials 20, 2311 (1999).
M. Geetha, A. Singh, R. Asokamani, and A. Gogia, Prog. Mater. Sci. 54, 397 (2009).
L. Le Guéhennec, A. Soueidan, P. Layrolle, and Y. Amouriq, Dent. Mater. 23, 844 (2007).
P.H. Pennekamp, J. Gessmann, O. Diedrich, B. Burian, M.A. Wimmer, V.M. Frauchiger, and C.N. Kraft, J. Orthop. Res. 24, 531 (2006).
J. Costerton, P.S. Stewart, and E. Greenberg, Science 284, 1318 (1999).
R.A. Weinstein and R.O. Darouiche, Clin. Infect. Dis. 33, 1567 (2001).
I. Uçkay, P. Hoffmeyer, D. Lew, and D. Pittet, J. Hosp. Infect. 84, 5 (2013).
V. Antoci Jr, C.S. Adams, J. Parvizi, H.M. Davidson, R.J. Composto, T.A. Freeman, E. Wickstrom, P. Ducheyne, D. Jungkind, and I.M. Shapiro, Biomaterials 29, 4684 (2008).
M. Kazemzadeh-Narbat, J. Kindrachuk, K. Duan, H. Jenssen, R.E. Hancock, and R. Wang, Biomaterials 31, 9519 (2010).
C.R. Rathbone, J.D. Cross, K.V. Brown, C.K. Murray, and J.C. Wenke, J. Orthop. Res. 29, 1070 (2011).
E.M. Hetrick and M.H. Schoenfisch, Chem. Soc. Rev. 35, 780 (2006).
D. Campoccia, L. Montanaro, P. Speziale, and C.R. Arciola, Biomaterials 31, 6363 (2010).
G.M. Harbers, K. Emoto, C. Greef, S.W. Metzger, H.N. Woodward, J.J. Mascali, D.W. Grainger, and M.J. Lochhead, Chem. Mater. 19, 4405 (2007).
A. Shimotoyodome, T. Koudate, H. Kobayashi, J. Nakamura, I. Tokimitsu, T. Hase, T. Inoue, T. Matsukubo, and Y. Takaesu, Antimicrob. Agents Chemother. 51, 3634 (2007).
Y. An, G. Stuart, S. McDowell, S. McDaniel, Q. Kang, and R. Friedman, J. Orthop. Res. 14, 846 (1996).
B. Jose, V. Antoci Jr, A.R. Zeiger, E. Wickstrom, and N.J. Hickok, Chem. Biol. 12, 1041 (2005).
J. Price, A. Tencer, D. Arm, and G. Bohach, J. Biomed. Mater. Res. 30, 281 (1996).
A. Russell, U. Tattawasart, J.-Y. Maillard, and J. Furr, Antimicrob. Agents Chemother. 42, 2151 (1998).
D.A. Wininger and R.J. Fass, Antimicrob. Agents Chemother. 40, 2675 (1996).
L. Harris, L. Mead, E. Müller-Oberländer, and R. Richards, J. Biomed. Mater. Res. A 78, 50 (2006).
I. Banerjee, R.C. Pangule, and R.S. Kane, Adv. Mater. 23, 690 (2011).
L. Zhao, P.K. Chu, Y. Zhang, and Z. Wu, J. Biomed. Mater. Res. B Appl. Biomater. 91, 470 (2009).
B. Gottenbos, H.C. van der Mei, F. Klatter, P. Nieuwenhuis, and H.J. Busscher, Biomaterials 23, 1417 (2002).
M. Mrksich and G.M. Whitesides, Annu. Rev. Biophys. 25, 55 (1996).
M. Mohorčič, I. Jerman, M. Zorko, L. Butinar, B. Orel, R. Jerala, and J. Friedrich, J. Mater. Sci. Mater. Med. 21, 2775 (2010).
J.C. Love, L.A. Estroff, J.K. Kriebel, R.G. Nuzzo, and G.M. Whitesides, Chem. Rev. 105, 1103 (2005).
L.G. Harris and R.G. Richards, Injury 37, S3 (2006).
K.A. Brogden, Nat. Rev. Microbiol. 3, 238 (2005).
A. Giuliani, G. Pirri, and S.F. Nicoletto, Cent. Eur. J. Biol. 2, 1 (2007).
M. Zasloff, Nature 415, 389 (2002).
K. Reddy, R. Yedery, and C. Aranha, Int. J. Antimicrob. Agents 24, 536 (2004).
M. Pasupuleti, A. Schmidtchen, and M. Malmsten, Crit. Rev. Biotechnol. 32, 143 (2012).
H. Jenssen, P. Hamill, and R.E. Hancock, Clin. Microbiol. Rev. 19, 491 (2006).
A.B. Ingham and R.J. Moore, Biotechnol. Appl. Biochem. 47, 1 (2007).
K. Hilpert, M.R. Elliott, R. Volkmer-Engert, P. Henklein, O. Donini, Q. Zhou, D.F. Winkler, and R.E. Hancock, Chem. Biol. 13, 1101 (2006).
C.D. Fjell, H. Jenssen, K. Hilpert, W.A. Cheung, N. Pante, R.E. Hancock, and A. Cherkasov, J. Med. Chem. 52, 2006 (2009).
Z. Jiang, A.I. Vasil, J.D. Hale, R.E. Hancock, M.L. Vasil, and R.S. Hodges, J. Pept. Sci. 90, 369 (2008).
M. Sarikaya, C. Tamerler, A.K.-Y. Jen, K. Schulten, and F. Baneyx, Nat. Mater. 2, 577 (2003).
D. Campoccia, L. Montanaro, and C.R. Arciola, Biomaterials 34, 8533 (2013).
P.H. Kwakman, A.A. te Velde, C.M. Vandenbroucke-Grauls, S.J. Van Deventer, and S.A. Zaat, Antimicrob. Agents Chemother. 50, 3977 (2006).
O. Etienne, C. Picart, C. Taddei, Y. Haikel, J. Dimarcq, P. Schaaf, J. Voegel, J. Ogier, and C. Egles, Antimicrob. Agents Chemother. 48, 3662 (2004).
P. Appendini and J. Hotchkiss, J. Appl. Polym. Sci. 81, 609 (2001).
Z. Yan, M.L. Snead, and C. Tamerler, Nanomedicine 11, 431 (2015).
S.S. Socransky and A.D. Haffajee, Periodontology 2000 28, 12 (2002).
D. Campoccia, L. Montanaro, and C.R. Arciola, Biomaterials 27, 2331 (2006).
F. Costa, I.F. Carvalho, R.C. Montelaro, P. Gomes, and M.C.L. Martins, Acta Biomater. 7, 1431 (2011).
S.R. Meyers, X. Khoo, X. Huang, E.B. Walsh, M.W. Grinstaff, and D.J. Kenan, Biomaterials 30, 277 (2009).
M. Yoshinari, T. Kato, K. Matsuzaka, T. Hayakawa, and K. Shiba, Biofouling 26, 103 (2010).
H. Yazici, H. Fong, B. Wilson, E. Oren, F. Amos, H. Zhang, J. Evans, M. Snead, M. Sarikaya, and C. Tamerler, Acta Biomater. 9, 5341 (2013).
E.E. Oren, C. Tamerler, D. Sahin, M. Hnilova, U.O.S. Seker, M. Sarikaya, and R. Samudrala, Bioinformatics 23, 2816 (2007).
Z. Lu, K.S. Murray, V. Van Cleave, E.R. LaVallie, M.L. Stahl, and J.M. McCoy, Nat. Biotechnol. 13, 366 (1995).
C. Tamerler, D. Khatayevich, M. Gungormus, T. Kacar, E.E. Oren, M. Hnilova, and M. Sarikaya, J. Pept. Sci. 94, 78 (2010).
C. Tamerler and M. Sarikaya, Acta Biomater. 3, 289 (2007).
U.O.S. Seker, B. Wilson, S. Dincer, I.W. Kim, E.E. Oren, J.S. Evans, C. Tamerler, and M. Sarikaya, Langmuir 23, 7895 (2007).
C.D. Fjell, H. Jenssen, W.A. Cheung, R.E. Hancock, and A. Cherkasov, Chem. Biol. Drug Des. 77, 48 (2011).
S. Chaudhury, S. Lyskov, and J.J. Gray, Bioinformatics 26, 689 (2010).
K.T. Simons, C. Kooperberg, E. Huang, and D. Baker, J. Mol. Biol. 268, 209 (1997).
P. Bradley, K.M. Misura, and D. Baker, Science 309, 1868 (2005).
W. Kabsch and C. Sander, Biopolymers 22, 2577 (1983).
J.W. Grzymala-Busse and W. Rzasa, Fund. Inform. 100, 99 (2010).
N.E. Shepherd, H.N. Hoang, G. Abbenante, and D.P. Fairlie, J. Am. Chem. Soc. 131, 15877 (2009).
Acknowledgements
The authors gratefully acknowledge financial support from National Institute of Health (NIH)—Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), Musculoskeletal Tissue Engineering Section 7R21AR062249-03 and University of Kansas New Faculty General Research Fund (NFGRF) as well as National Institute of Dental and Craniofacial Research Grant DE13045.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yucesoy, D.T., Hnilova, M., Boone, K. et al. Chimeric Peptides as Implant Functionalization Agents for Titanium Alloy Implants with Antimicrobial Properties. JOM 67, 754–766 (2015). https://doi.org/10.1007/s11837-015-1350-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-015-1350-7