Abstract
Purpose
To test the hypothesis that epiphysiodesis made with radiofrequency ablation (RFA) is a safe procedure that disrupts the growth plate without damaging the adjacent joint articular cartilage.
Methods
RFA epiphysiodesis was done during 8 min in vivo in 40 growing pig tibia physis. In addition, three tibiae were ablated for 16 min and three more for 24 min. As a burned cartilage reference, six tibiae were ablated on the joint articular cartilage for 8 min. After the procedure, the animals were terminated and the tibiae were harvested. Magnetic resonance imaging (MRI) was done ex vivo to evaluate the joint articular cartilage in all samples. We used T1-weighted, T2-weighted, and water content sequences under a 1.5 T magnetic field.
Results
On the burned articular cartilage, intensity changes were observed at MRI. We found no evidence of articular cartilage damage on the 40 8-min RFA procedures. The tibiae ablated for 16 min and 24 min showed intact joint cartilage.
Conclusions
Epiphysiodesis using RFA is safe for the adjacent articular cartilage. This study shows that RFA can be done safely in the growing physis of pigs, even with triple duration procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cartilage has a nonlinear thermomechanical behavior [1]. Díaz et al. report cartilage changes at increasing temperatures: at 50 °C, thermal injury is observed; when 56 °C is reached, there is a sharp decrease in cell variability associated to compromised cell membranes, which lead to cell death at 60 °C [2]. Chondrocytes are particularly sensitive to temperature. Articular cartilage damage is permanent and progressive. Degeneration and swelling of the cartilage reduce its ability to absorb compressive load, resulting in crepitus and pain [3]. Chondral defects are seen in 60 % of all knee arthroscopy procedures with injury to articular cartilage, causing progressive and permanent damage. Heat starts decreasing glycosaminoglycan, metalloproteinase 13, interleukin 1, and nitric oxide, which cause significant changes to the cartilage [4]. Chondrocyte death in only one-fourth of the articular cartilage thickness causes irreversible, extended damage [5]. Epiphysiodesis using radiofrequency ablation (RFA) has been reported to be successful in animal models. It can induce growth arrest in both small [6] and large species [7]. This procedure induces thermal damage and coagulates necrosis in the physis. Because of the proximity of the physis and the epiphysis, there is a theoretical possibility to damage the articular cartilage when performing epiphysiodesis using RFA. Ablation devices have been deployed in treatment settings requiring tissue preservation, like debridement chondroplasty and modeling. Because of this, several articular cartilage models have been designed to study tissue preservation and perturbation [8]. The risk of damaging the adjacent joint articular cartilage when performing epiphysiodesis using RFA has not been reported, to our knowledge.
Aim: to test the hypothesis that epiphysiodesis made with RFA is a safe procedure that disrupts the growth plate without damaging the adjacent joint articular cartilage.
Methods
Ethics
The study was conducted in compliance with the Danish Animal Research Guidelines. Ethical Committee authorization was not needed; this was an acute, nonsurvival study.
Design
The study was divided into four sub-studies: positive controls (burned cartilage), study A (8-min-long ablation, 20 animals, 40 bilateral procedures), study B (16-min-long ablation, three animals, six bilateral procedures), and study C (24-min-long ablation, three animals, six bilateral procedures).
Burned cartilage reference parameters
The proximal tibia (plateau) articular cartilage was ablated for 8 min in six animals (12 bilateral ablations). The obtained data were used as reference for damaged cartilage. The procedure was done laterally and medially.
Physis RFA
Ablation was done directly in the tibia physis for 8 min (study A), 16 min (study B), and 24 min (study C). The procedure was done laterally and medially.
Sample size
For study A, we expected an intact articular cartilage in the adjacent joint to the ablation (tibiae plateau) visible on magnetic resonance imaging (MRI) in 100 % of the cases. The desired power was 90 % and α = 0.05. Thus, 20 animals were included in the study. Two procedures were done on each animal (medial and lateral ablation). In addition, six animals were included as positive controls (burned cartilage) and six were included for studies B (three bilateral ablations) and C (three bilateral ablations). Study B represents a double-time ablation (16 min) and study C a triple-time ablation (24 min).
Animals
Healthy, skeletally immature female pigs from the Yorkshire–Landrace–Duroc race were used in all the procedures. All the animals were 15 weeks old and 40.2 kg on average (37.6–44.8 kg).
Methods
Magnetic resonance
Images were obtained ex vivo at room temperature (21 °C). All the treated tibiae were harvested after the procedure and kept frozen at −18 °C. All MRI was done at the MR Research Center, Aarhus University Hospital, Skejby. A whole-body scanner (Siemens Magnetom Avanto 1.5 Tesla, Erlangen, Germany) was used to acquire the following four image sets sequentially:
-
1.
Spin echo (SE) T1-weighted imaging using the following parameters: echo time (TE) 20 ms, repetition time (TR) 800 ms, slice thickness 3 mm, field-of-view (FOV) 120 × 120, matrix 384 × 268, and two signal averages (NEX).
-
2.
Water content in the tissue was calculated from T1 maps. Both the two flip angle method and inversion recovery sequences were applied. The two flip angle method (gradient echo sequence with flip1 5°, flip2 30°, TE 1.61 ms, TR 15 ms, slice thickness 4 mm, NEX 3, FOV 180 × 101 mm, and matrix 256 × 256) is fast, with an acquisition time of 14 min. The inversion recovery method (11 inversion times from 200 to 2200 ms, slice thickness 4 mm, FOV 200 × 200, matrix 256 × 256) has an acquisition time of 34 min.
-
3.
Proton density weighted fast SE sequence (TE 41 ms, TR 2000 ms, echo train length 7, slice thickness 2 mm, FOV 180 × 101 mm, matrix 448 × 314, NEX 2).
-
4.
T2-weighted fast SE sequence (TE 73 ms, TR 4610 ms, echo train length 1, slice thickness 2.0 mm, FOV 160 × 130 mm, matrix 256 × 192, NEX 6).
Anesthesia and medication
For premedication, the animals received an intramuscular injection with ketaminol (5 mg/kg; S-ketamine, Pfizer, Berlin, Germany) and midazolam (0.5 mg/kg; Hypnomidate, Janssen-Cilag, Beerse, Belgium). The animals were intubated and anesthesia was maintained with a gas mixture of sevoflurane (Sevorane, Abbott Laboratories, Chicago, IL, USA) and O2 60 %. For analgesia, an intravenous infusion of fentanyl (0.025 mg/kg/h; Haldid, Janssen-Cilag, Beerse, Belgium) was maintained during the procedure.
Epiphysiodesis
All the procedures were done using a 7-mm, 18 G radiofrequency probe without cooling (Cool-tip™ RFA system, Covidien AG, USA). Ablation power (watts) was corrected constantly during the procedure to maintain the temperature between 92 and 98° C. Impedance fluctuated between 90 and 700 Ω during the procedure.
Operation technique
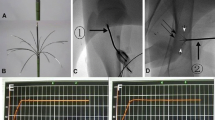
Under fluoroscopic guidance (Fig. 1), the proximal tibiae growth plate was identified and a penetration cannula (Bonopty® Bone Biopsy System, AprioMed AB, Uppsala, Sweden) was inserted 90° from the vertical plane towards the growth plate. From the skin through the soft tissue, a stylet was used and when the periosteum was reached, it was drilled into the growth plate. Then, the radiofrequency probe was inserted 1 cm into the growth plate and the ablation was done.
Euthanasia
After the ablation, under general anesthesia, the animals received a lethal intravenous injection of pentobarbital 0.5 ml/kg (200 ml/kg) and the tibiae were harvested.
Analysis
Magnetic resonance images were analyzed using Syngo FastView software (Siemens©, AG, Berlin and Munich 2004–2008). The proximal tibial joint cartilage was identified and then analyzed for discontinuity and intensity changes (Fig. 2). Also, the T1 value of the cartilage (Fig. 3) was measured, seeking changes above 25 % using Siswin software version 0.9 (Steffen Ringgaard© 2008).
Results
Physis-ablated tibiae (studies A, B, and C)
None of the ablated tibiae showed intensity changes on MRI (Fig. 2). T1 values from the articular cartilage showed a mean difference of 3 % (2–5 %) between the different MR sections. We did not find any difference between the tibiae that were ablated for 8, 16, and 24 min. All the values were similar (Table 1).
Burned cartilage group
A hyper-intensity was observed at the ablation area (Fig. 4). T1 values from these areas were increased by 30 % on average (20–42 %) (Fig. 5; Table 1).
Discussion
This nonsurvival study is based on a previously published protocol that results in growth arrest without giving the animal discomfort or complications during the follow-up [7]. Early changes caused by thermal damage to the articular cartilage are described by Meister et al. [9]. They report that thermal lesions of articular cartilage can be observed as “shrinkage” surrounded by edema. MR is accepted to analyze cartilage damage and it can detect early changes that result in degeneration [10]. After analysis using MR, we did not find any changes in the procedures where the physis was ablated. We could not identify shrinkage of the articular cartilage in the group that was ablated directly on the cartilage, but edema was evident and it was measured indirectly using MR T1 values [11]. In the present study, we chose a porcine model because the bone biology resembles humans and the distance between the physis and the articular cartilage is closely equal to humans [12]. Subjective evaluation of MRI both in T1-weighted and T2-weighted images can suggest tissue changes. In colored water content images (Fig. 6), these changes are easier to identify. However, an objective analysis is more accurate and can be obtained from T1 mapping. This allows the quantification of damage and usage of reference parameters. The water content allows it to be known if the cells are compromised or they suffered damage. Damaged cell membranes lead to cell death. Thermal damage affects mainly membranes. Such changes directly affect the water content immediately [3]. Damaged membranes lead to edema, which causes an influx of calcium. This phenomenon can be observed even in temperatures of 50 °C, which would not cause cellular death but would suppress the ability of chondrocytes to remodel, leading to permanent damage [13].
MR images from the physis ablated 8, 16, and 24 min looked similar on MRI, where hyper-intensities or morphological changes could not be identified. From the T1 values, we concluded that edema was not present at the articular cartilage after the procedure. An isolative effect from the bone is described by Caffey et al. [14]. On a study in fresh cartilage, they conclude that radiofrequency probes produce significant cellular death after 1 and 3 s. However, this does not happen when the probe is placed 1 mm from the cartilage. Our porcine model is close to biological human characteristics, but a validation for human tissue should be done from our translational research. Also, during childhood, the epiphysis size varies. Our model does not consider this size variation, which could be a limitation. Another limitation of our study could be that we only included coronal images, although we believe that it does not affect our analysis.
In conclusion, RFA of the tibia physis is a safe procedure for the adjacent joint cartilage. The energy and heat is contained at the ablation site. We found no evidence of joint cartilage damage based on macroscopy, MRI intensity, and water content.
References
Baum OI, Soshnikova YM, Sobol EN, Korneychuk AY, Obrezkova MV, Svistushkin VM, Timofeeva OK, Lunin VV (2011) Laser reshaping of costal cartilage for transplantation. Lasers Surg Med 43:511–515. doi:10.1002/lsm.21077
Díaz SH, Nelson JS, Wong BJF (2003) Rate process analysis of thermal damage in cartilage. Phys Med Biol 48:19–29
Kosy JD, Schranz PJ, Toms AD, Eyres KS, Mandalia VI (2011) The use of radiofrequency energy for arthroscopic chondroplasty in the knee. Arthroscopy 27(5):695–703. doi:10.1016/j.arthro.2010.11.058
Horton D, Anderson S, Hope NG (2014) A review of current concepts in radiofrequency chondroplasty. ANZ J Surg 84(6):412–416. doi:10.1111/ans.12130
Kääb MJ, Bail HJ, Rotter A, Mainil-Varlet P, apGwynn I, Weiler A (2005) Monopolar radiofrequency treatment of partial-thickness cartilage defects in the sheep knee joint leads to extended cartilage injury. Am J Sports Med 33(10):1472–1478
Ghanem I, El Hage S, Diab M, Saliba E, Khazzaka A, Aftimos G, Dagher F, Kharrat K (2009) Radiofrequency application to the growth plate in the rabbit: a new potential approach to epiphysiodesis. J Pediatr Orthop 29(6):629–635. doi:10.1097/BPO.0b013e3181b2bae7
Shiguetomi-Medina JM, Rahbek O, Abood AA, Stødkilde-Jørgensen H, Møller-Madsen B (2014) Thermal epiphysiodesis performed with radio frequency in a porcine model. Acta Orthop 85(5):538–542. doi:10.3109/17453674.2014.939014
Ganguly K, McRury ID, Goodwin PM, Morgan RE, Augé WK II (2010) Histopomorphic evaluation of radiofrequency mediated débridement chondroplasty. Open Orthop J 4:211–220. doi:10.2174/1874325001004010211
Meister J, Franzen R, Gavenis K, Zaum M, Stanzel S, Gutknecht N, Schmidt-Rohlfing B (2009) Ablation of articular cartilage with an erbium:YAG laser: an ex vivo study using porcine models under real conditions—ablation measurement and histological examination. Lasers Surg Med 41:674–685
Novakofski KD, Pownder SL, Koff MF, Williams RM, Potter HG, Fortier LA (2016) High-resolution methods for diagnosing cartilage damage in vivo. Cartilage 7(1):39–51. doi:10.1177/1947603515602307
Shiguetomi-Medina JM, Gottliebsen M, Kristiansen MS, Ringgaard S, Stødkilde-Jørgensen H, Rahbek O, Møller-Madsen B (2013) Water-content calculation in growth plate and cartilage using MR T1-mapping design and validation of a new method in a porcine model. Skeletal Radiol 42(10):1413–1419. doi:10.1007/s00256-013-1674-8
Aerssens J, Boonen S, Lowet G, Dequeker J (1998) Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology 139(2):663–670
Huber M, Eder C, Mueller M, Kujat R, Roll C, Nerlich M, Prantl L, Gehmert S (2013) Temperature profile of radiofrequency probe application in wrist arthroscopy: monopolar versus bipolar. Arthroscopy 29(4):645–652
Caffey S, McPherson E, Moore B, Hedman T, Vangsness CT Jr (2005) Effects of radiofrequency energy on human articular cartilage: an analysis of 5 systems. Am J Sports Med 33(7):1035–1039
Acknowledgments
We gratefully thank Covidien for facilitating the radiofrequency generator and providing technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study did not receive any external funding.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The study was conducted in compliance with the Danish Animal Research Guidelines, and accepted and authorized by the Danish Animal Experiment Committee (study J. Nr. 2008/561-329).
Conflict of interest
All the authors declare they have no conflict of interest.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Shiguetomi-Medina, J.M., Rahbek, O., Abood, A.A.H. et al. Does radiofrequency ablation (RFA) epiphysiodesis affect adjacent joint cartilage?. J Child Orthop 10, 359–364 (2016). https://doi.org/10.1007/s11832-016-0747-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11832-016-0747-3