Abstract
A study was conducted to determine rates of recapture of marked Agriotes obscurus (AO) males released in a grassy field on four occasions in the centre of pheromone trap squares with traps spaced 2.5, 5.0, 7.5 and 10.0 m apart. Concurrent catches of naturally occurring and naturally dispersed (wild) AO were also recorded. Catch of marked AO was highest during peak emergence when maximum weekly temperatures ranged from 15.7 to 17 °C, and rainfall was light. Mean catch in the 2.5–10.0 m trap squares was, respectively, 82.0, 79.0, 77.0 and 65.0%, which occurred within one week of release. Catch of marked AO were lower (range in 2.5–10.0 m squares: 74.5–50.5%) when released early in the activity period, due to lower temperatures and higher rainfall, and catch was protracted over 3 weeks. Catch during two releases under favourable weather late in the activity period was also reduced (range: 25–58%), likely due to loss of AO vigour and/or pheromone trap efficacy. Catch of wild AO was highest in traps spaced 7.5 or 10.0 m apart, suggesting independence of these traps, and between-trap competition at 2.5 and 5.0 m spacings. From these data, we estimated that an effective spacing for traps in mass trapping arrays would be approximately 6 m. Significantly higher catch of marked or wild AO occurred in traps that were upwind in all trap squares, indicating wind direction should be considered in structuring the placement of trap arrays in mass trapping programs in non-farmed headland areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Three invasive species of wireworms from Europe, Agriotes obscurus (L.), A. lineatus (L.) and A. sputator (L.), have become major pests of several key crops (i.e., potatoes, carrots, cereals, corn) in the provinces of British Columbia (BC), Prince Edward Island and Nova Scotia, Canada (van Herk and Vernon 2021a; Vernon and van Herk 2022). These species, which have larval life cycles in soil of 4–5 years, become permanently established in new agricultural areas primarily via the mobile adult click beetle stage. Since habitats containing grasses are preferred for click beetle oviposition and subsequent wireworm development, the non-farmed undisturbed headland areas surrounding arable fields are typically the first colonized. Once established, all life stages of one or more of these Agriotes species can be found, culminating in the annual production of click beetles that can repeatedly invade adjacent arable fields whenever suitable oviposition hosts are planted (i.e., pasture, cereal and forage crops) (Brian 1947).
Once established in arable fields, the management of wireworms generally defaults to the use of insecticides applied prophylactically to crop seed (i.e., cereals, forages, potatoes) or to soil at the time of planting (i.e., granules or in-furrow sprays). Although effective, the use of insecticides for wireworm control since the 1940s has often been controversial due to earlier products being highly residual in soil (i.e., organochlorines such as aldrin, heptachlor and lindane) or being highly toxic to humans and other organisms in the environment (i.e., organophosphates and carbamates) (Elliott et al. 1996; Vernon and van Herk 2022). A global consequence of these concerns has been the gradual removal of the most effective insecticides used for wireworm control, and many of the newer classes of insecticides, although providing crop protection through repulsion (e.g., pyrethroids: van Herk and Vernon 2007; Vernon et al. 2009; van Herk et al. 2015) or long term morbidity (e.g., neonicotinoids, diamides: van Herk et al. 2015), do not result in significant reductions in wireworm populations (Vernon et al. 2009; van Herk et al. 2018a; 2021b). As a result, populations of various wireworm species are on the increase throughout the Holarctic despite the use of these contemporary insecticide treatments.
Since the turn of the current century, the re-emergence of wireworms as key economic pests of many crops has prompted considerable research into alternative controls, including: biological (i.e. microbial and nematode); cultural (i.e. allelopathic crops, strategic rotations); and semiochemical methods (mating disruption and mass trapping) (Parker and Howard 2001; Ritter and Richter 2013; Barsics et al. 2013; Traugott et al. 2015; Vernon and van Herk 2022). As has been the case with insecticides, the aim of these alternative methods has been to target various elaterid life stages present within arable fields. Unfortunately, many of these alternative control methods are less practical, more expensive or less efficacious than insecticides, and treated fields are always prone to chronic reinvasion by click beetles emerging within the surrounding non-farmed headlands (Traugott et al. 2015; Furlan et al. 2016; Vernon and van Herk 2022). Alternatively, one or more control methods that target these non-farmed headland reservoirs should be given greater consideration, since these areas occupy only a fraction of farmland area and would not interfere with in-field farming activities (Vernon et al. 2014b; Vernon and van Herk 2022). Of the alternatives mentioned, semiochemical methods such as mass trapping and/or mating disruption, which generally involve species-specific pheromones, are strong candidates, since effects on non-target vertebrates and invertebrates occurring in non-farmed areas are minimized (Vernon and van Herk 2022).
In the case of the invasive species in Canada mentioned above (A. obscurus, A. lineatus and A. sputator), pheromones and trap devices have been developed (Furlan et al. 2002; Furlan and Tóth 2007; Tóth et al. 2003; Vernon and Tóth 2007; van Herk et al. 2018b, 2021c, 2022), and pilot mass trapping studies have been conducted both ‘in-field’ (Balkov 1991; Hicks and Blackshaw 2008; Sufyan et al. 2011), and in ‘non-farmed headlands’ (Vernon et al. 2014a, b). In the non-farmed headland studies, parallel transects of A. obscurus (AO) or A. lineatus (AL) pheromone traps with rows and traps spaced 3 m apart along corridors of grassy headland recaptured 85.6% of mark-released AO and 77.8% of marked AL with arrays of their respective pheromone traps, mostly within the first week of release. In arrays of mixed AO and AL traps, recapture rates were 77.8% and 83.3%, respectively (Vernon et al. 2014a). These results, and those from concurrent trapping studies with wild, non-marked AO and AL (Vernon et al. 2014b), suggested that mass trapping might be used to remove sufficient male AO and AL beetles to significantly reduce mating and associated oviposition in nonfarmed habitats.
Although promising, the spacing of 3 m between traps in these mass trapping arrays was considered at that time to be too expensive for multi-year deployment (i.e., 4 years for AO and AL) in non-farmed areas. Therefore, before mass trapping can even be considered as a valid strategy for reducing AO and AL populations in non-farmed habitats, greater spacing between traps would be required to reduce costs while maintaining rates of male removal equal to the 3 m trap arrays tested previously. The data obtained in our preliminary studies, as well as data reported on trap spacing for AO and AL by others (Hicks and Blackshaw 2008; Sufyan et al. 2011), suggested that trap spacings between 3 and 10 m should be tested in arrays for AO and AL in permanent grassy habitats. The studies conducted herein investigated the efficacy of traps placed in square, 4 trap arrays with traps spaced 2.5, 5.0, 7.5 and 10 m apart in trapping wild and marked populations of AO males on 4 occasions in a grassy field in 2014. In addition, the effect of wind direction on the efficacy of various traps in each array was investigated.
Materials and methods
Experimental design
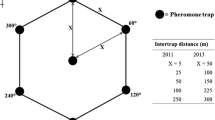
Trap spacing studies were conducted in an area of long-term permanent grass surrounding a field of highbush blueberries at the Agassiz Research and Development Centre (ARDC, Agriculture and Agri-Food Canada) in Agassiz (49° 14′ 50″ N; 121° 45′ 45″ W; WGS84), situated at the eastern end of the lower Fraser Valley of BC (Fig. 1). The traps used were Vernon Beetle Traps (VBTs, described by Vernon 2004) used in earlier mark recapture trials and baited with A. obscurus pheromone bubble caps (Contech Inc., Delta, BC) (Vernon et al. 2014a, b). Traps were arranged in 4-trap squares, with traps in squares spaced 2.5, 5.0, 7.5, or 10.0 m apart (Fig. 1). Trap squares were arranged in randomized linear blocks, with at least 12 m separating traps between squares in replicates, and 15 m separating trap squares from the nearest replicate. Each trap was positioned in the middle of 40 cm square areas of removed sod (depressions of about 8–10 cm relative to the top of surrounding mowed grass). Since VBTs are square, flow-through traps open only on opposing sides, the trap orientation was approximated with the flow-through axis pointing toward the middle of each square trap array (Fig. 1B). Traps were positioned relative to the estimated predominant wind direction in the area (approx. south to north), such that one trap in each square was always upwind (Fig. 1B). The actual predominant wind directions during the studies were later determined from an Environment and Climate Change Canada weather station at the ARDC (World Meteorological Organization Identifier: 71,113, approx. 300 m from the study site). Since AO adults appear to be most active at temperatures > 10 °C (van Herk, personal observation), predominant wind directions were determined for each study period using wind direction measurements only during hours when temperatures were > 10 °C and wind speeds > 5 km/h. Treatments (trap spacings) and replicates (n = 5) were kept the same between consecutive studies. The grass was kept short (about 7 cm high) throughout the study by mowing with a ride-on lawn mower between trapping sessions (2–3 weeks).
Square trap arrays used to determine the effectiveness of pheromone traps spaced 2.5, 5.0, 7.5 and 10.0 m apart (n = 5 replicates) in a grass field (A) in capturing marked Agriotes obscurus (AO) males released in the centre of each square (B), as well as naturally dispersed wild AO beetles. Releases were done on 4 occasions in 2014, with AO beetles marked different colors that were randomized in squares within replicates in each release. Traps in squares were positioned according to the N, E, S, and W cardinal directions (B)
Mark-release method
Mark-release was used to determine how effective the various trap spacings were in capturing A. obscurus males released in the centre of each square (Fig. 1B). Releases were conducted from 16 April to 7 May (Study 1), 7–21 May (Study 2), 3–16 June (Study 3), and 18 June to 9 July (Study 4). Within one week prior to each release, wild male AO beetles were collected in additional VBTs in the general region of the studies and stored at room temperature (c. 16–18 °C) in well ventilated Plexiglas cages (30 × 100 × 100 cm). The day before each release, beetles were marked on their pronotums with various acrylic paints (Golden Artist Colors, Inc., New Berlin, NY, USA 13411-3616). The paints used were pre-tested on lab populations and had no detrimental effects on click beetle health or behaviour. During storage, beetles were fed with freshly cut apple slices every 2–3 days. On the day of release, 20 healthy and well-marked AO beetles were placed in 10 cm diameter Petri dishes and released in the centre of each trap square. The Petri dishes were opened and immediately covered with 15 × 15 cm plywood lids that were elevated approx. 1 cm above the dishes such that beetles were released under shade. Four colors were used for each beetle release, with different colored beetles randomly assigned to each trap square in a replicate, and the same color colors not released in adjacent squares. On the day following each release, Petri dishes were examined and any unreleased beetles due to death or morbidity were counted. Thereafter, walking within each trap square was prohibited during each release period.
Traps were inspected on a weekly basis following each release on two (releases 2 and 3) or three occasions (releases 1 and 4). Following each release, traps were emptied into plastic containers labelled according to date, trap square (spacing), trap location in squares (N, S, E, W) and replicate, placed in a 4 °C cooler, and numbers of colored and wild beetles counted within 2 days of collection.
Statistical analysis
Data were analyzed with generalized linear model (Proc GENMOD), using a log-link function and a negative binomial distribution, using separate analyses per beetle release. Model factors were ‘trap spacing’, ‘trap position’, and ‘treatment’. Pairwise comparisons between either trap spacings or trap positions were done using the ‘lsmeans’ statement with Tukey’s adjustment. All analyses were performed in SAS Enterprise Guide v.7.1 (SAS Institute, Cary, NC, USA). For each release date, analyses were performed on both the total number of wild or marked beetles captured, and on weekly captures of these.
Results
Trap spacing
Release 1 (16 April–7 May)
This 3-week release-recapture period was characterized by low mean daily air temperatures (wks. 1, 2 and 3, respectively, 10.1, 11.8 and 14.2 °C), and high weekly precipitation (respectively, 59.9, 49.4 and 30.3 mm) (Fig. 2A). The release coincided with the major upswing and peak of wild AO emergence in the area (Fig. 2B).
Mean daily temperature and rainfall from 16 April to 9 July, 2014, during which time four releases of marked A. obscurus males were conducted in a field of grass in Agassiz, BC (A). Population trends (mean ± SEM) of wild AO beetles caught in pheromone traps in the area during this time period are shown (B)
Marked AO captures
At first inspection (23 April), it was noted that a small number of beetles (6/400 released) had not left the Petri dishes, and these were removed from the field. The mean (SD) percent recapture rates of marked beetles in the 2.5, 5.0, 7.5 and 10.0 m trap squares at this date were, respectively, 63.2 (7.3), 52.1 (7.6), 26.2 (12.3) and 26.7 (24.5)% (Chi = 16.5, P = 0.0009, Tukey’s = A, AB, B, B, respectively). This trend was reversed at second inspection (30 April), where captures in the 2.5–10.0 squares were, respectively, 6.2 (4.3), 9.1 (10.8), 24.1 (17.0) and 16.0 (14.3)% (Chi = 9.69, P = 0.021, Tukey’s = B, AB, AB, A), which persisted into the third inspection (7 May) with recaptures of 5.2 (6.4), 5.1 (6.1), 12.2 (4.6) and 8.0 (15.2)% (Chi = 3.17, P = 0.37). Although significantly more catches occurred in the 2.5 and 5.0 squares during the first week of release, there were no significant differences in recapture rates among the 2.5–10.0 m squares overall (respectively, 74.5 (9.7), 66.3 (2.2), 62.4 (16.1) and 50.7 (13.3)%; Chi = 5.39, P = 0.15) (Table 1 and Fig. 3A). It was also noted that some marked beetles had left the trap squares of their releases and were captured in other squares which were a minimum of 12 m away. This occurred with 4, 9 and 10 marked AO over the 3 inspection dates, respectively, amounting to 5.8% of the total beetles released, and these beetles are not included in Table 1 or Figs. 3 and 5.
Wild AO captures
In contrast to the release of a finite number of marked AO in the centre of the various trap squares, the collection of unmarked, wild AO in these traps were from larger populations more uniformly distributed throughout the grassy study area. As such, wild beetles could access individual traps in the trap squares from all directions. In contrast to the recapture of significantly higher levels of marked beetles at first inspection (23 April), mean (SD) numbers of wild males taken in the 2.5–10.0 m trap squares were not significantly different (respectively, 19.5 (13.2), 15.1 (8.2), 23.4 (15.3) and 19.5 (15.0) AO per trap; Chi = 6.15, P = 0.10), suggesting trap squares were attracting wild beetles equally during that time. This trend began to change at second inspection, where numbers of wild AO were numerically highest in the 7.5 and 10.0 m trap squares (respectively, 22.2 (27.1), 23.9 (38.3), 33.0 (37.6) and 34.1 (37.5) AO per trap; Chi = 6.87, P = 0.08), which was even more pronounced at third inspection (respectively, 55.7 (52.9), 46.0 (44.1), 70.4 (52.9) and 61.7 (49.6) AO per trap; Chi = 9.56, P = 0.023, Tukey’s = AB, B, A, AB). When data were combined, catch was highest overall in the 7.5 m trap squares, followed by the 10.0 and 2.5 m spacings (respectively, 97.3 (88.5), 85.0 (83.7), 126.8 (97.3), 115.2 (97.2) AO per trap for 2.5–10.0 m spacings; Chi = 10.11, P = 0.018, Tukey’s = AB, B, A, AB) (Table 2 and Fig. 4A).
Release 2 (7–21 May)
This 2-week release-recapture period was characterized by higher mean daily air temperatures (wks. 1 and 2, respectively, 15.8 and 17.0 °C), and low precipitation (wks. 1 and 2, respectively, 13.0 and 2.6 mm) (Fig. 2A), and coincided with initially high but thereafter dropping wild AO population levels (Fig. 2B).
Marked AO captures
At first inspection (14 May), the mean (SD) percent recapture of marked beetles in the 2.5–10.0 m trap squares were, respectively, 82.0 (7.6), 79.0 (13.4), 77.0 (15.2) and 65.0 (13.7)%, with no significant differences occurring between trap squares (Chi = 3.43, P = 0.33). At second inspection, only one additional marked beetle was taken (10.0 m square), indicating that catch in all trap squares was accelerated under the warmer and drier conditions of this release relative to Release 1 (Table 1, Figs. 2A, 3B). Higher numbers of marked beetles left the trap squares in Release 2 relative to Release 1, with 50 and 3 marked AO being captured in other squares over the two inspection dates, respectively, amounting to 13.3% of the total beetles released.
Wild AO captures
At first inspection (14 May), mean (SD) numbers of wild AO were numerically lower in the 2.5 and 5.0 m squares relative to the 7.5 and 10.0 m squares (respectively, 40.7 (36.1), 36.4 (30.8), 52.2 (47.8) and 54.9 (57.0) AO per trap; Chi = 3.52, P = 0.32). At second inspection, numbers of wild AO in the trap squares had generally dropped, with no significant differences occurring among the 2.5–10.0 m trap squares (respectively, 17.9 (12.9), 17.5 (10.9), 23.3 (20.2) and 20.4 (14.0) AO per trap; Chi = 2.43, P = 0.49). When inspections were pooled, the highest numbers of wild AO were caught in the 7.5 and 10.0 m trap squares (respectively, 58.6 (48.1), 53.9 (32.5), 75.5 (62.2) and 75.3 (68.5) AO per trap. Chi = 2.81, P = 0.42), which was also observed in weeks 2 and 3 of Release 1 (Table 2, Fig. 4B).
Releases 3 (3–16 June) and 4 (18 June–9 July)
Weather during Release 3 was similar to Release 2, with high mean daily air temperatures (wks. 1 and 2, respectively, 16.3 and 14.7 °C), and low precipitation (wks. 1 and 2, respectively, 4.2 and 11.1 mm) (Fig. 2A). Traps had been removed from plots at the end of Release 2 (21 May) and re-installed on 2 June just before Release 3. Grass had been cut on 30 May to a height of about 7 cm.
Weather during Release 4 was similar to Release 3, with high mean daily air temperatures (wks. 1–3, respectively, 15.3, 18.8 and 18.4 °C), and low weekly precipitation (wks. 1–3, respectively, 9.4, 10.4 and 2.2 mm) (Fig. 2A). Traps that were removed at the end of Release 3 (16 June) during mowing were re-installed on 18 June just before Release 4.
Marked AO captures
At first inspection of Release 3 (10 June), the mean (SD) recapture rates of marked beetles in the 2.5–10.0 m trap squares were, respectively, 50.0 (12.2), 57.0 (16.0), 39.0 (13.9) and 22.0 (14.4)%, with only 4.0 (2.2), 1.0 (2.2), 2.0 (2.7) and 3.0 (4.5)% recaptured at second inspection (16 June). When data were combined, significant differences occurred in catch among the 2.5–10.0 m trap squares (Chi = 10.45, P = 0.02, Tukey’s = A, A, AB, B, respectively), with highest catches once again in the 2.5–5.0 m squares (Table 1 and Fig. 3C). During the two inspections, 23 and 10 marked beetles, respectively, were captured in other squares, amounting to 8.3% of the total beetles released.
In Release 4, the percent recapture of marked beetles at first inspection (25 June) in the 2.5–10.0 m trap squares were, respectively, 43.0 (13.5), 27.0 (14.8), 35.0 (17.0) and 23.0 (15.7)%, with 1.0 (2.2), 0.0, 3.0 (4.5) and 2.0 (2.7)% AO recaptured at second inspection (2 July), and 2.0 (2.7)% recaptured in the 7.5 m square at final inspection (July 9). When data were combined, there were no significant differences in catch among the 2.5–10.0 trap squares (Chi = 8.0, P = 0.046, Tukey's NS). During the three inspections, 43, 12 and 1 marked beetles were captured in other squares, respectively, amounting to 14.0% of the total beetles released. The lower recovery of marked beetles in Releases 3 and 4 relative to Releases 1 and 2 are coincident with the declining vigour of AO populations during that time (Fig. 2B).
Wild AO captures
At first inspection of Release 3, mean (SD) numbers of wild AO caught in the 2.5–10.0 m trap squares were considerably lower relative to Releases 1 and 2 (respectively, 6.6 (4.6), 8.8 (9.8), 9.8 (10.4) and 8.8 (10.3) AO per trap) and even more so at second inspection (respectively, 3.1 (2.5), 4.4 (3.2), 4.5 (3.3) and 3.0 (2.4) AO per trap) (Fig. 4C). There were no significant differences in catch of wild AO among trap spacings in the individual or pooled inspections (respectively, 9.7 (4.7), 13.1 (10.8), 14.3 (12.3) and 11.8 (12.0) AO per trap; Chi = 3.83, P = 0.28) (Table 2, Fig. 4C).
In Release 4, significant differences in catch of wild AO per trap among the 2.5–10.0 m squares occurred at first inspection (respectively, 2.1 (1.5), 3.9 (4.2), 2.9 (2.6) and 2.0 (2.5); Chi = 10.52, P = 0.015, Tukey’s = AB, A, AB, B, respectively), second inspection (respectively, 1.9 (1.4), 2.9 (3.7), 5.0 (5.8) and 3.0 (3.0); Chi = 13.51, P = 0.0037, Tukey’s = B, B, A, B, respectively), third inspection (respectively, 0.6 (0.9), 1.1 (0.9), 2.0 (2.5) and 0.9 (1.4). Chi = 16.61, P = 0.0009, Tukey’s = B, AB, A, AB), and combined inspections (respectively, 4.6 (2.4), 7.8 (7.6), 9.8 (8.9) and 5.8 (5.8); Chi = 18.4, P = 0.0004, Tukey’s = B, AB, A, B). It is interesting to note that the highest catches were in the 5.0 and 7.5 m trap spacings, which was also observed in Release 3 (Table 2, Fig. 4D).
Trap directional preferences
During Releases 1–4, the predominant wind directions at temperatures 10 °C or higher and wind speed > 5 km/h were, respectively, 228°, 227°, 224°, 230°, or generally from the Southwest (Fig. 1B), with the percentage of time wind was recorded from these directions being, respectively, 39, 53, 67 and 62%.
Marked AO captures
In Release 1, the mean (SD) proportion of marked AO in traps positioned at the North, East, South and West (N, E, S, W) cardinal directions of all 2.5–10.0 m trap squares combined were, respectively, 18.9 (12.4), 17.2 (10.2), 26.8 (17.2) and 37.1 (18.6)% of the total captures, with significantly higher captures in West than North and East traps (Chi = 21.74, P = 0.0001, Tukey’s = A, A, AB, B, respectively, N, E, S, W) (Fig. 5A). Subsequently, significantly higher captures were observed in South traps relative to other positions in Release 2 (Chi = 84.19, P = 0.0001, Tukey’s = B, B, A, B), Release 3 (Chi square = 31.21, P = 0.0001, Tukey’s = C, B, A, B) and Release 4 (Chi = 46.88, P = 0.0001, Tukey’s = B, B, A, B) (respectively, Fig. 5B-D). These trends in trap position preferences did not differ significantly (Table 1) among the 2.5–10 m trap squares.
Mean (SD) proportion of marked A. obscurus beetles recaptured per pheromone trap after releases on 4 occasions in a grassy field in 2014 (A–D). Traps were placed in squares spaced 2.5, 5.0, 7.5 and 10.0 m apart in N, E, S, and W cardinal directions. The prevailing wind direction during the 4 releases was from the Southwest
Wild AO captures
As was generally observed with the marked beetle catches, the highest numbers of wild AO were taken in the South traps (Fig. 6). Significant differences among N, E, S, W trap positions, however, were only observed in Release 1 (Chi = 11.12, P = 0.01, Tukey’s = AB, B, A, AB, respectively) (Fig. 6A) and Release 4 (Chi = 28.35, P = 0.0001, Tukey’s = B, AB, A, B, respectively) (Fig. 6D). As observed with the marked AO catches, trends in trap position preferences with wild AO did not differ among the 2.5–10 m trap squares.
Mean (SD) proportion of wild male A. obscurus beetles recaptured per pheromone trap after releases on 4 occasions in a grassy field in 2014 (A–D). Traps were placed in squares spaced 2.5, 5.0, 7.5 and 10.0 m apart in N, E, S, and W cardinal directions. The prevailing wind direction during the 4 releases was from the Southwest
Discussion
Trap spacing efficacy: marked AO
In previous work (Vernon et al. 2014a, b), Vernon Beetle Traps (VBTs) for A. obscurus (AO) were placed 3 m apart in six 2 × 15 trap arrays centred along 45 m long by 10 m wide sections of a grassy dyke in Delta, BC. Of these arrays, three contained only AO traps and three contained both AO and A. lineatus (AL) traps in alternating rows. At the peak period of AO male activity in the area, beetles were released randomly throughout each 45 m long trap array, and captures of marked (Vernon et al. 2014a) and wild AO (Vernon et al. 2014b) were recorded on a weekly basis throughout the complete activity period. During this study, 85.6% and 77.8% of marked AO were recaptured in, respectively, pure AO and mixed AO and AL arrays, mostly within the first week of release. In addition, wild AO populations were significantly reduced in the pure and mixed trap arrays, and it was concluded that AO mating encounters would have been significantly reduced due to a combination of timely male removal and likely disorientation of untrapped males due to overlapping pheromone saturation in the arrays. Based on this preliminary work, a 75–86% recapture rate of marked AO in the current trap square studies is a reasonable target to match the efficacy of the 3 m trap arrays used in the previous study.
In the current study, VBTs were presented in squares spaced 2.5, 5.0, 7.5 and 10.0 m apart, and marked AO were released in the centre of each trap square (respectively, 1.76, 3.53, 5.30 and 7.01 m from AO release points to nearest traps) on four occasions over the principal AO activity period (16 April to 9 July). Of these, Release 2 best matches the single marked AO release conducted in the previous study (Vernon et al. 2014a) in terms of weather conditions and peak AO activity. In the 2.5 m trap squares (closest spacing to the 3.0 m trap arrays in the previous study) 82% of marked AO were recaptured, mostly within the first week of release (Fig. 3B), which falls within the 75–85% recapture target. Where the current and previous studies differ, however, is that marked AO in the current study were released in the centre of the 2.5 m square (1.76 m to nearest trap) as opposed to random releases along the 3.0 m trap arrays (0—2.12 m to nearest trap). As such, the majority of randomly released beetles in the previous study would have had a shorter distance to travel to the nearest trap. In addition, marked AO that left the 3.0 m trap squares could have been caught in other adjacent traps along the 2 × 15 trap arrays. Numbers recaptured in 2.5 m squares in the current study, therefore, likely underestimate the recapture rates that would be expected with random releases or natural emergence along longer trap arrays. Release 2 of the current study also showed that competitive rates of marked AO recapture occurred in 5.0 m and 7.5 m trap squares (respectively 79 and 77%), which, for reasons stated above, would likely produce higher catch rates with longer trap arrays and randomly dispersed populations. Traps spaced 10.0 m apart caught the fewest marked AO (66%), suggesting the targeted 75–86% recapture efficacy diminishes at trap spacings somewhere above 7.5 m.
The current studies further expand on the previous study in that the recapture efficacy of marked AO among the 2.5–10.0 m trap spacings varied considerably among the 4 releases (Fig. 4A–D). Recapture rates in Release 1 for example, coinciding with the upswing of AO emergence, occurred more gradually over a three-week period with lower overall catches in all trap spacings (respectively, 74, 66, 62 and 51% recapture, Fig. 2A) relative to Release 2. This difference is attributed to the cooler temperatures and higher rainfall occurring during Release 1 (Fig. 2A), which would have slowed the movement of marked AO and/or reduced the release and dissipation of pheromone in the trap squares. Supporting data for this was observed in the delayed collection of marked AO in the 7.5 and 10.0 m squares relative to the 2.5 and 5.0 m trap squares (Fig. 3A). Recapture rates were also reduced in all trap squares during Releases 3 and 4 despite favourable weather conditions occurring, which is attributed to populations of AO declining in numbers and/or mating vigor at that time, and/or a possible reduction in release of AO pheromone from traps. The reduction in trapping efficacy of marked AO later in the activity period is of low importance however, in that the majority of wild male AO emerging naturally within mass trapping arrays would likely have been captured by that time, as was shown in the previous study (Vernon et al. 2014b).
Between trap competition: wild AO
In addition to the desired 75–86% recapture rate, another postulated requirement for successful mass trapping is that competition between traps in trapping arrays should occur, but without reducing total catch in individual traps (Lanier 1990). Evidence to support this requirement among the 2.5–10.0 m trap squares was not provided in the mark-release studies, due to the finite numbers of marked AO released in the centres of each square, and the strong directional recapture bias in the southernmost traps (discussed below, Fig. 3A–D). However, concurrent catches of wild AO that were dispersed randomly in high numbers throughout the study area did suggest between trap competition was occurring between certain trap spacings. During the first 2 release periods when populations were highest, pooled numbers of wild AO per trap in the 2.5 and 5.0 m trap squares were lower than in the 7.5 and 10.0 trap squares (mean (SD) respectively, 4.5 (3.8), 4.0 (3.1), 5.8 (4.4) and 5.4 (4.6) AO/trap/day; Chi = 7.11, P = 0.068). These data suggest that between-trap competition was occurring in trap squares spaced 2.5 and 5.0 m apart, which is desirable, but that reductions in catch per trap (about 25%) was also occurring relative to the larger trap squares. The data also suggest that traps in both the 7.5 and 10.0 m squares, which caught similar numbers of wild AO, were functioning independently of each other, and that competition caused by pheromone overlap between these traps was likely not occurring to the extent observed in the 2.5 and 5.0 m squares. What this indicates is that maximum catch per trap and between-trap competition will likely occur somewhere between traps spaced 5.0 and 7.5 m apart. Assuming that increases in catch of wild AO per trap between 5.0 and 7.5 m squares is linear, a conservative spacing of about 6.0 m should increase catch per trap above the 2.5 and 5.0 m squares without sacrificing between-trap competition.
Directional trap bias: marked and wild AO
The current study clearly demonstrated that marked AO released in the centre of trap squares of all spacings generally responded significantly to traps in the upwind position during the 4 releases (Fig. 5A–D). This trend was also observed with wild AO, where traps in the upwind position consistently and sometimes significantly caught the highest numbers (Fig. 6A–D). In the case of wild AO, all traps would have been upwind of the more dispersed AO distribution within and on all sides of the trap squares, but the most upwind (south and west) traps would have been most attractive to those wild AO occurring within the trap squares. These data conflict with other studies where the movement of marked AO and AL released at increasing distances along various cardinal directional transects radiating from single pheromone traps did not show directional bias (Hicks and Blackshaw 2008; Sufyan et al. 2013). In these other studies, releases were made in open fields of permanent pasture (Hicks and Blackshaw 2008) or in fields of grass-clover (Sufyan et al. 2013), where crop height was higher than the pheromone traps used (Yatlorf traps, Csalomon, Budapest, Hungary) in contrast to the mowed field surrounding trap squares in the current study. The lack of directional bias in the Hicks and Blackshaw (2008) study was postulated to be due to the high grass surrounding the traps, which would have resulted in pheromone being disseminated more by ‘unbiased diffusion’ rather than a ‘wind-directed plume’. This would likely have also been the case in the grass-clover field studies by Sufyan et al. (2013). With single traps in tall grass, therefore, pheromone dissipation would be more multi-directional, especially during times of low or variable wind movement (Hicks and Blackshaw 2008). In contrast, wind-directed plumes were likely occurring in the current study due to the shorter grass surrounding the traps, and pheromone dissipation into trap squares by the upwind south trap would have outcompeted the other traps leading to the higher catches observed. It is also notable that in an indoor setting, Blackshaw et al. (2018a) observed male AO to move downwind, even in the presence of pheromone, when wind speeds were > c. 20 km/h. It is possible that the avoidance of wind gusts may have contributed to the lower than anticipated captures in the westernmost traps.
Optimization of AO mass trapping efficacy and economics
The data presented herein expands and improves on our previous mass trapping studies (Vernon et al. 2014a, b) in ways that could improve the efficacy and economic viability of mass trapping strategies to reduce reservoir populations of AO in headland habitats (Blackshaw et al. 2018b). With respect to efficacy, the current data suggest that between-trap and between-row spacings of 6 m along grassy headlands will provide similar and timely removal of wild AO populations to the 3 m spacings used in our previous studies. The data also showed that knowledge of the prevailing wind direction during the most active periods of AO emergence should be considered during placement of trap arrays in headlands. For example, in 10 m wide grassy headlands (typical of agricultural areas in BC), two rows of traps spaced 6 m apart should be positioned to provide optimal wind-directed coverage of the headland area. Where no wind effects are present, as along leeward- and downward-sloping headlands, the 6 m trap arrays can be oriented with both rows about 2 m from their respective headland edges. Where the prevailing wind passes across an exposed headland, the array can be shifted about 1 m upwind to better collect upwind populations.
Our estimate of 6 m trap spacings required for mass trapping in ‘headlands’ varies considerably from other estimates for ‘in-field’ mass trapping developed elsewhere. Hicks and Blackshaw (2008) estimated that mass trapping of AO in fields would require about 15 traps per ha with between-trap spacing of about 25 m, and Sufyan et al. (2013) estimated that 25 traps per ha, with between-trap spacing of about 20 m would be required to reduce AO male populations in fields by 50%. Our current and previous studies (Vernon et al. 2014a, b) however, suggest that the requirement for between-trap competition (Lanier 1990) in traps spaced 20 or 25 m apart would be unlikely, especially where unbiased pheromone diffusion occurs in actively growing pasture or clover, and the likelihood of male/female encounters would remain high. Any reduction in rapid and uniform capture of AO males resulting in reduced mass trapping efficacy (i.e., continued mating and oviposition) is undesirable due to the high cost involved with in-field and/or headland trapping required over a 4-year period.
The current studies focussed on A. obscurus, which is often present in headland areas with other pest elaterids including AL and AS. In BC, for example, both AO and AL are found together in almost all agricultural fields in the lower Fraser Valley (Vernon et al. 2001; Blackshaw and Vernon 2006; Vernon and Tóth 2007), and mass trapping would need to include both species. In the work of Hicks and Blackshaw (2008), it was found that AL disperses more rapidly than AO, and they determined that mass trapping of AL in fields would require even fewer traps than AO (i.e. 10 versus 15 traps/ha). In our earlier work, we also found equivalent catch of marked AL and AO males in 3 m trap arrays (Vernon et al. 2014a). These studies indicate that increasing inter-trap distance in arrays to 6 m for AO should also be effective for AL. Since we have found that placing AO and AL pheromone traps 1.5 m apart has no effect on individual trap efficacy (Vernon et al. 2014a), rows of AO traps spaced 6 m apart can include alternating AL traps midway between AO traps.
With respect to the economics of mass trapping, the proposed increased distance between traps in headland arrays makes mass trapping a more affordable strategy for the long-term management of AO. Since the goal of mass trapping elaterids in headland areas is to functionally eliminate the reservoir populations that annually invade arable fields, and since the cost and effort involved are protracted over 4 years (the life history for AO), such programs must be conducted on an area-wide basis to justify the effort. Assuming this, we can estimate the relative costs of mass trapping in agricultural landscapes of various dimensions, where mass trapping could be done either in-field (as proposed by Balkov 1991; Hicks and Blackshaw 2008; and Sufyan et al. 2013) and/or in surrounding headlands (as proposed by Vernon et al. 2014a, b). For example, an area containing 9, 5 ha square arable fields in a 3 × 3 matrix with each field surrounded by a 10 m wide grassy headland, would require 1,842 AO headland traps versus 12,477 in-field traps spaced 6 m apart in various arrays. The cost of materials would involve the traps (VBT = $6.40 USD) and lures (AO lures = $3.00), the former lasting over 4 years and the latter having to be purchased annually. Over 4 years of trapping, the total cost of mass trapping (traps + lures) would be $33,896 in headlands versus $117,283 in-field, or respectively $8,474 versus $29,320 per year. Assuming successful mass trapping in headlands or in-field would ultimately result in protection of arable fields from economic wireworm damage, the cost of trapping materials per ha would amount to $188/ha/year with headland trapping versus $652/ha/year with in-field trapping. In addition, by removing wireworm populations from headland reservoir areas, it would take at least an additional 4-years post-trapping for wireworm populations to begin recovery to levels able to invade surrounding fields. Since the cost of chemical control of wireworm infested potato fields can be high (i.e., the cost of Thimet 20G is about $160 USD per ha and requires expensive application equipment), the amortized cost of mass trapping in headlands, and the continued wireworm crop protection post-trapping which requires no in-field chemical intervention, is a reasonable alternative control option.
Conclusions and future studies
Since these studies were conducted (2014), VBTs are no longer available, but have been replaced in North America by the Vernon Pitfall Trap® (VPT, Intko Supply, Ltd., Chilliwack, BC, Canada) which, when baited with pheromone lures used in Yatlorf traps (Csalomon, Budapest, Hungary) provides equivalent or superior catch of AO and AL males to the VBT (van Herk et al. 2022). In addition, the cost of VPTs ($5.00 USD) is lower than VBTs and requires much less time to assemble, install and service in the field, making mass trapping even more economically feasible (van Herk et al. 2022).
Although promising, some additional research would be required to determine the effects of variations in headland vegetation and AO (and/or AL) population levels on mass trapping efficacy. Vegetation along headlands often varies in the presence and abundance of ground cover preferred for elaterid oviposition and wireworm feeding, and the unmanaged growth of vegetation concurrent with adult emergence could gradually change pheromone dissipation from initial wind-directed plumes (as experienced in the current studies) to unbiased diffusion (as discussed in Hicks and Blackshaw 2008). In addition, previous studies determined that opportunities for mating encounters in 3 × 3 m VBT arrays increased for AL where populations in headlands were high (Vernon et al. 2014b), which could be further intensified with trap arrays spaced further apart. The effects of these variables on mass trapping efficacy could be determined using release recapture studies of AO and/or AL adults along headlands with various vegetative characteristics and wild populations of increasing size,
Data availability
Data is available upon request.
References
Balkov VI (1991) Attractant traps for control of wireworms. Zashchita Rastenii (Moskova) 5:24–25
Barsics F, Haubruge E, Verheggen FJ (2013) Wireworms’ management: an overview of the existing methods, with particular regards to Agriotes spp. (Coleoptera: Elateridae). InSects 4:117–152. https://doi.org/10.3390/insects4010117
Blackshaw RP, Vernon RS (2006) Spatio-temporal stability of two beetle populations in non-farmed habitats in an agricultural landscape. J Appl Ecol 43:680–689. https://doi.org/10.1111/j.1365-2664.2006.01167.x
Blackshaw RP, Van Herk WG, Thiebaud F (2018a) Determination of Agriotes obscurus (Coleoptera: Elateridae) sex pheromone attraction range using target male behavioural responses. Agric For Entomol 20:228-233
Blackshaw RP, Vernon RS, Thiebaud F (2018b) Large scale Agriotes spp. click beetle (Coleoptera: Elateridae) invasion of crop land from field margin reservoirs. Agric for Entomol 20:51–61. https://doi.org/10.1111/afe.12228
Brian MV (1947) On the ecology of beetles of the genus Agriotes with special reference to A. obscurus. J Anim Ecol 16:210–224. https://doi.org/10.2307/1496
Elliott JE, Langelier KM, Mineau P, Wilson LK (1996) Poisoning of bald eagles and redtailed hawks by carbofuran and fensulfothion in the Fraser delta of British Columbia, Canada. J Wildl Dis 32:486–491. https://doi.org/10.7589/0090-3558-32.3.486
Furlan L, Tóth M (2007) Occurrence of click beetle pest spp. (Coleoptera, Elateridae) in Europe as detected by pheromone traps: survey results of 1998–2006. IOBC/WPRS Bull 30:19–25
Furlan L, Tóth M, Parker WE, Ivezic M, Pančič S, Brmeź M, Dobrinčić R, Igrc Barčić J, Muresan F, Subchev M et al (2002) The efficacy of the new Agriotes sex pheromone traps in detecting wireworm population levels in different European countries. IWGO-Newsletter 23:16–17
Furlan L, Contiero B, Chiarini F, Colauzzi M, Sartori E, Benvegnu I, Fracasso F, Giandon P (2016) Risk assessment of maize damage by wireworms (Coleoptera:Elateridae) as the first step in implementing IPM and in reducing the environmental impact of soil insecticides. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-016-7692-z
Hicks H, Blackshaw RP (2008) Differential responses of three. Agriotes click beetle species to pheromone traps. Agric For Entomol 10:443–448
Lanier GN (1990) Principles of attraction–annihilation: mass trapping and other means. In: Ridgway RL, Silverstein RM, Inscoe MN (eds) Behaviour-modifying chemicals for insect management. Marcel Dekker Inc., New York
Parker WE, Howard JJ (2001) The biology and management of wireworms (Agriotes spp.) on potato with particular reference to the UK. Agric for Entomol 3:85–98
Ritter C, Richter E (2013) Control methods and monitoring of Agriotes wireworms (Coleoptera: Elateridae). J Plant Dis Prot 120:4–15. https://doi.org/10.1007/BF03356448
Sufyan M, Neuhoff D, Furlan L (2011) Assessment of the range of attraction of pheromone traps to Agriotes lineatus and Agriotes obscurus. Agric For Entomol 13:313–319
Sufyan M, Neuhoff D, Furlan L (2013) Effect of male mass trapping of Agriotes pheromone traps on wireworm abundance and potato tuber damage. Bull Insectology 66:135–142
Tóth M, Furlan L, Yatsynin VG, Ujváry I, Szarukán I, Imrei Z, Tolasch T, Francke W, Jossi W (2003) Identification of pheromones and optimization of bait composition for click beetle pests (Coleoptera: Elateridae) in Central and Western Europe. Pest Manag Sci 59:417–425. https://doi.org/10.1002/ps.629
Traugott M, Benefer C, Blackshaw RP, van Herk WG, Vernon RS (2015) Biology, ecology, and control of elaterid beetles in agricultural land. Annu Rev Entomol 60:313–334. https://doi.org/10.1146/annurev-ento-010814-021035
van Herk WG, Vernon RS (2007) Soil bioassay for studying behavioral responses of wireworms (Coleoptera: Elateridae) to insecticide-treated wheat seed. Environ Entomol 36:144–1449. https://doi.org/10.1603/0046-225X(2007)36[1441:SBFSBR]2.0.CO;2
van Herk WG, Vernon RS, Vojtko B, Snow S, Fortier J, Fortin C (2015) Contact behaviour and mortality of wireworms exposed to six classes of insecticide applied to wheat seed. J Pest Sci 88:717–739. https://doi.org/10.1007/s10340-015-0697-4
van Herk WG, Labun TJ, Vernon RS (2018a) Efficacy of diamide, neonicotinoid, pyrethroid, and phenyl pyrazole insecticide seed treatments for controlling the sugar beet wireworm, Limonius californicus (Coleoptera: Elateridae), in spring wheat. J Entomol Soc B C 115:86–100
van Herk WG, Vernon RS, Borden JH (2018b) A pheromone-baited pitfall trap for monitoring Agriotes spp. click beetles (Coleoptera: Elateridae) and other soil surface insects. J Entomol Soc B C 115:101–103
van Herk WG, Vernon RS, Acheampong S, Otani JK, Uloth K (2021a) Distribution of two European elaterids, Agriotes obscurus and A. lineatus in British Columbia: New records, and potential implications of their dispersal. J Asia Pac Entomol 24:288–694. https://doi.org/10.1016/j.aspen.2021.06.001
van Herk WG, Vernon RS, Goudis L, Mitchell T (2021b) Broflanilide, a meta-diamide insecticide seed treatment for protection of wheat and mortality of wireworms (Agriotes obscurus) in the field. J Econ Entomol 114:161–173. https://doi.org/10.1093/jee/toaa239
van Herk WG, Vernon RS, Richardson J, Richardson M, Beaton A (2021c) Evaluation of pheromone traps and lures for trapping male Agriotes sputator (Coleoptera: Elateridae) beetles in eastern Canada. Fla Entomol 104:42–50. https://doi.org/10.1653/024.104.0107
van Herk WG, Vernon RS, Borden JH, Ryan K, Mercer G (2022) Comparative evaluation of pitfall traps for click beetles (Coleoptera: Elateridae). J Econ Entomol 115:582–591. https://doi.org/10.1093/jee/toab259
Vernon RS (2004) A ground-based pheromone trap for monitoring Agriotes lineatus and A. obscurus (Coleoptera: Elateridae). J Entomol Soc B C 101:83–84
Vernon RS, Tóth M (2007) Evaluation of pheromones and a new trap for monitoring Agriotes lineatus and Agriotes obscurus in the Fraser valley of British Columbia. J Chem Ecol 33:345–351. https://doi.org/10.1007/s10886-006-9217-6
Vernon RS, van Herk WG (2022) Wireworms as pests of potato. In: Alyokhin A, Rondon S, Gao Y (eds) Insect pests of potato: global perspectives on biology and management, 2nd edn. Academic Press, Elsevier, pp 103–148
Vernon B, LaGasa E, Philip H (2001) Geographic and temporal distribution of Agriotes obscurus and A. lineatus (Coleoptera: Elateridae) in British Columbia and Washington as determined by pheromone trap surveys. J Entomol Soc B C 98:257–265
Vernon RS, van Herk WG, Clodius M, Harding C (2009) Wireworm management I: stand protection versus wireworm mortality with wheat seed treatments. J Econ Entomol 102:2126–2136. https://doi.org/10.1603/029.102.0616
Vernon RS, van Herk WG, Blackshaw RP, Shimizu Y, Clodius M (2014a) Mark-release-recapture of Agriotes obscurus and A. lineatus with pheromone traps in a permanent grassland population reservoir. Agric for Entomol 16:217–226. https://doi.org/10.1111/afe.12045
Vernon RS, Blackshaw RP, van Herk WG, Clodius M (2014b) Mass trapping wild Agriotes obscurus and Agriotes lineatus males with pheromone traps in a permanent grassland population reservoir. Agric for Entomol 16:227–239. https://doi.org/10.1111/afe.12058
Acknowledgements
We thank our dedicated summer students Andrew Boxwell, Romain Richard, Hafsa Salihue, Tessa Ramburn, Amber Burnett, Victoria Piollat, Marie Tissot, Damien Rabusseau, and Crystal Chan, for their enthusiastic and very capable assistance with all aspects of the beetle trapping work.
Funding
Open access funding provided by Agriculture & Agri-Food Canada
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Handling editor: Diana la Forgia.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van Herk, W.G., Vernon, R.S. Capture of wild and marked Agriotes obscurus in pheromone traps according to distance, wind direction and date of trapping. Arthropod-Plant Interactions 17, 529–542 (2023). https://doi.org/10.1007/s11829-023-09961-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-023-09961-0