Abstract
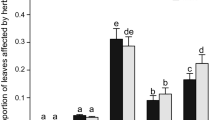
Frequently, female plants allocate more resources to reproductive structures and defense-related secondary compounds in comparison with male plants that invest more resources to growth, reflecting trade-offs between reproduction, growth and defense. Therefore, differences in herbivory can be expected between genders. In this study, over two years, we analyzed the differences in plant chemical defense, nutritional quality, plant size and herbivory between genders in the dioecious tree, Spondias purpurea in a Mexican tropical dry forest. We estimated the total leaf area and the area consumed by folivory using a digital image of each leaf. The nutritional quality was estimated as water content, and the concentration of chlorophyll and total nonstructural carbohydrates. The secondary metabolites analyzed were total content of soluble phenolics, flavonoids, protein precipitation capacity of tannins, gallotannins, soluble proanthocyanidins, hydrolyzable tannins and ellagitannins. Our results differ from most of studies that analyze the differential herbivory patterns in dioecious plants. We found that female trees had higher levels of herbivory than male trees of S. purpurea. In the same way, female trees showed higher size and nutritional quality than males, while chemical defense was higher in male trees. The higher percentage of folivory in female trees of S. purpurea is associated with greater nutritional quality and lower chemical defenses. Our results show that male-biased herbivory might not be universal in dioecious species. Therefore, studies of fitness components affected by herbivory are necessary to understand the evolution of dioecy and the importance of herbivores as selective agents on breeding system features.




Similar content being viewed by others
References
Agren J, Danell K, Elmqvist T, Ericson L, Hjältén J (1999) Sexual dimorphism and biotic interactions. In: Geber MA, Dawson TE, Delph LF (eds) Gender and sexual dimorphism in flowering plants. Springer, Berlin, pp 217–246
Ahman I (1997) Growth, herbivory, and disease in relation to gender in Salix viminalis L. Oecologia 111:61–68
Ashman T (1994) A dynamic perspective on the physiological cost of reproduction in plants. Am Nat 144:300–316
Ashman TL (2000) Pollinator selectivity and its implications for the evolution of dioecy and sexual dimorphism. Ecology 81:2577–2591
Ashman TL (2002) The role of herbivores in the evolution of separate sexes from hermaphroditism. Ecology 83:1175–1184
Ashman TL (2009) Sniffing out patterns of sexual dimorphism in floral scent. Funct Ecol 23:852–862
Avila-Sakar G, Romanov CA (2012) Divergence in defence against herbivores between males and females of dioecious plant species. Int J Evol Biol 2012:1–16
Bañuelos MJ, Sierra M, Obeso JR (2004) Sex, secondary compounds and asymmetry. Effects on plant-herbivore interaction in a dioecious shrub. Acta Oecol 25:151–157
Basset Y (1996) Local communities of arboreal herbivores in Papua New Guinea: predictor of insect variables. Ecology 77:1906–1919
Basset Y (2001) Communities of insect herbivores foraging on saplings versus mature trees of Pourouma bicolour (Cecropiaceae) in Panama. Oecologia 129:253–260
Bawa KS (1980) Evolution of dioecy in flowering plants. Ann Rev Ecol Syst 11:15–39
Berenbaum MR (1995) Chemical defense: theory and practice. Proc Natl Acad Sci-Biol 92:2–8
Boecklen WJ, Price PW, Mopper S (1990) Sex and drugs and herbivores: sex-biased herbivory in arroyo willow (Salix lasiolepis). Ecology 71:581–588
Boecklen WJ, Mopper S, Price PW (1994) Sex-biased herbivory in arroyo willow: are there general patterns among herbivores? Oikos 71:267–272
Brown VK, Lawton JH (1991) Herbivory and the evolution of leaf size and shape. Philos Trans R Soc B 333:265–272
Bullock SH (1984) Biomass and nutrient allocation in a neotropical dioecious palm. Oecologia 63:426–428
Bullock SH (1988) Rasgos del ambiente físico y biológico de Chamela, Jalisco, México. Folia Entomol Mexicana 77:5–17
Bullock SH (1992) Seasonal differences in nonstructural carbohydrates in two dioecious monzon-climate trees. Biotropica 24:140–145
Bullock SH, Solís-Magallanes JA (1990) Phenology of canopy trees of a tropical deciduous forest in Mexico. Biotropica 22:22–35
Campbell D (2000) Experimental tests of sex-allocation theory in plants. Trends Ecol Evol 15:227–232
Castro-Esau KL, Sánchez-Azofeifa A, Rivard B, Wright SJ, Quesada M (2006) Variability in leaf optical properties of mesoamerican trees and the potential for species classification. Am J Bot 93:517–530
Cepeda-Cornejo V, Dirzo R (2010) Sex-related differences in reproductive allocation, growth, defense and herbivory in three dioecious neotropical palms. PLoS ONE 5:e9824
Charnov EL (1982) The theory of sex allocation. Princeton University Press, Princeton
Cibils AF, Swift DM, Hart RH (2003) Female-biased herbivory in four wing saltbush browsed by cattle. J Range Manage 56:47–51
Coley PD (1982) Rates of herbivory on different tropical trees. In: Leigh EG, Rand AS Jr, Windsor DM (ed) The ecology of a tropical forest. Smithsonian Institution Press, Washington, pp 123–132
Coley PD, Barone JA (1996) Herbivory and plant defenses in tropical forests. Ann Rev Ecol Syst 27:305–335
Coley PD, Bryant JP, Chapin III FS (1985) Resource availability and plant anti-herbivore defense. Science 230:895–899
Cornelissen T, Stiling P (2005) Sex biased herbivory: a meta-analysis of the effects of gender on plant-herbivore interactions. Oikos 111:488–500
Correia O, Diaz Barradas MC (2000) Ecophysiological differences between male and female plants of Pistacia lentiscus L. Plant Ecol 149:131–142
Craig TP, Itami JK, Price PW (1989) A strong relationship between oviposition preference and larval performance in a shoot-galling sawfly. Ecology 70:1691–1699
Cuevas-Reyes P, Quesada M, Siebe C, Oyama K (2004) Spatial patterns of herbivory by gall-forming insects: a test to the soil fertility hypothesis in a Mexican tropical dry forest. Oikos 107:181–189
Cuevas-Reyes P, Quesada M, Oyama K (2006) Abundance and leaf damage caused by gall-inducing insects in a Mexican tropical dry forest. Biotropica 38:107–115
Cuevas-Reyes P, Oyama K, González-Rodríguez A, Fernandes GW, Mendoza-Cuenca L (2011) Contrasting herbivory patterns and leaf fluctuating asymmetry in Heliocarpus pallidus between different habitat types within a Mexican tropical dry forest. J Trop Ecol 27:383–391
Danell K, Hjältén J, Ericson L, Elmkvist T (1991) Vole feeding on male and female willow shoots along a gradient of plant productivity. Oikos 62:145–152
Dawson TE, Bliss LC (1989) Patterns of water use and the tissue water relations in the dioecious shub, Salix arctica: the physiological basis for habitat partitioning between the sexes. Oecologia 79:332–343
Dawson TE, Geber MA (1999) Dimorphism in physiology and morphology. In: Geber MA, Dawson TE, Delph LF (eds) Gender and sexual dimorphism in flowering plants. Springer, Berlin, pp 175–215
De Jong TJ (1995) Why fast-growing plants do not bother about defense. Oikos 74:545–548
Delph LF (1999) Sexual dimorphism in life history. In: Geber MA, Dawson TE, Delph LF (eds) Gender and sexual dimorphism in flowering plants. Springer, Berlin, pp 149–173
Diaz Barradas MC, Correia O (1999) Sexual dimorphism, sex ratio and spatial distribution of male and female shrubs in the dioecious species Pistacia lentiscus L. Folia Geobotanica 34:163–174
Doyle RD, Grodowitz M, Smart RM, Owens C (2002) Impact of herbivory by Hydrellia pakistanae (Diptera: Ephydridae) on growth and photosynthetic potential of Hydrilla verticillata. Biol Control 24:221–229
Feeny P (1970) Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51:565–581
Feeny P (1976) Plant apparency and chemical defense. Recent Adv Phytochem 10:1–40
Filip V, Dirzo R, Maass JM, Sarukhan J (1995) Within-and among-year variation in the levels of herbivory on the foliage of trees from a Mexican tropical deciduous forest. Biotropica 27:78–86
Frankie GW, Baker H, Opler P (1974) Comparative phenological studies of trees in tropical wet and dry forests in the lowlands of Costa Rica. J Ecol 62:881–899
Freeman DC, McArthur ED (1982) A comparison of twig water stress between males and females of six species of desert shrubs. For Sci 28:304–308
Fritz RS, Crabb BA, Hochwender CG (2000) Preference and performance of a gall-inducing sawfly: a test of the plant vigor hypothesis. Oikos 89:555–563
García-Robledo C (2005) Comparison between two methods to measure herbivory. Is herbivory in the neotropics more severe than we thought? Rev Biol Trop 53:111–114
Gehring JL, Monson RK (1994) Sexual differences in gas exchange and response to environmental stress in dioecious Silene latifolia (Caryophyllaceae). Am J Bot 81:166–174
Hagerman AE (1987) Radial diffusion method for determining tannin in plant extracts. J Chem Ecol 13:437–449
Hagerman AE, Zhao Y, Johnson S (1997) Methods for determination of condensed and hydrolyzable tannins. In: Shahadi F (ed) ACS symposium series 662. American Chemical Society, Washington, DC, pp 209–222
Hartzfeld PW, Forkner R, Hunter MD, Hagerman AE (2002) Determination of hydrolyzable tannins (gallotannins and ellagitannins) after reaction with potassium iodate. J Agric Food Chem 50:1785–1790
Hendricks BJ, Collier BD (2003) Effects of sex and age of a dioecious tree, Forchammeria pallida (Capparaceae) on the performance of its primary herbivore Murgantia varicolor (Hemiptera: Pentatomidae). Ecol Res 18:247–255
Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. Q Rev Biol 67:283–335
Hiscox JD, Israelstam GF (1979) Method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334
Hogan KP, García MB, Cheeseman JM, Loveless MD (1998) Inflorescence photosynthesis and investment in reproduction in the dioecious species Aciphylla glaucescens (Apiaceae). New Zeal J Bot 36:653–660
Horvitz CC, Schemsky DW (2002) Effects of plant size, leaf herbivory, local competition ecology and fruit production on survival, growth and future reproduction of a neotropical herb. J Ecol 90:279–290
Houle G (1999) Nutrient availability and plant gender influences on the short-term compensatory response of Salix planifolia spp. planifolia to simulated leaf herbivory. Can J For Res 29:1841–1846
Ibarra-Manríquez G, Oyama K (1992) Ecological correlates of reproductive traits of Mexican rain forest trees. Am J Bot 79:383–394
Inoue KH, Hagerman AE (1988) Determination of gallotannin with rhodanine. Anal Biochem 169:363–369
Jing SW, Coley PD (1990) Dioecy and herbivory: the effect of growth rate on plant defense in Acer negundo. Oikos 58:369–377
Karban R, Baldwin IT (1997) Induced responses to herbivory. University of Chicago Press, Chicago
Kursar TA, Coley PD (2003) Convergence in defense syndromes of young leaves in tropical rainforest. Biochem Syst Ecol 31:929–949
Laporte MM, Delph LF (1996) Sex specific physiology and source-sink relations in the dioecious plant Silene latifolia. Oecologia 106:63–72
Lott E, Bullock SH, Solís-Magallanes JA (1987) Floristic diversity and structure of upland and arroyo forest of coastal Jalisco. Biotropica 19:228–235
Lowman MD (1984) An assessment of techniques for measuring herbivory: is rain forest defoliation more intense than we thought? Biotropica 16:264–268
Macia JM, Barfod AS (2000) Economic botany of Spondias purpurea (Anacardiaceae) in Ecuador. Econ Bot 54:449–458
Marquis RJ, Newell EA, Villegas AC (1997) Nonstructural carbohydrates accumulation and use in understory rain forest shrub and relevance for the impact of leaf herbivory. Funct Ecol 5:636–643
McCall AC (2007) Leaf damage and gender but not flower damage affect female fitness in Nemophila menziesii (Hydrophyllaceae). Am J Bot 94:445–450
Niesenbaum RA (1992) The effects of light environment on herbivory and growth in the dioecious shrub Lindera benzoin (Lauraceae). Am Midl Nat 128:270–275
Obeso JR (2002) The cost of reproduction in plants. New Phytol 155:321–348
Opler PA, Frankie GH, Baker HG (1980) Comparative phenological studies of treelet and shrubs species in tropical wet and dry forests in the lowlands of Costa Rica. J Anim Ecol 68:167–188
Oyama K, Dirzo R (1988) Biomass allocation in the dioecious tropical palm Chamaedorea tepejilote and its life history consequences. Plant Species Biol 3:27–33
Oyama K, Dirzo R (1991) Ecological aspects of the interaction between Chamaedora tepejilote, a dioecious palm and Calyptocephala marginipenis, an herbivorous beetle, in a Mexican rain forest. Principes 35:86–93
Pascual-Alvarado E, Cuevas-Reyes P, Quesada M, Oyama K (2008) Interactions between galling insects and leaf-feeding insects: the role of plant phenolic compounds and their possible interference with herbivores. J Trop Ecol 24:329–336
Price PW (1991) The plant vigor hypothesis and herbivore attack. Oikos 62:244–251
Ribeiro SP, Pimenta HR, Fernandes GW (1994) Herbivory by chewing and sucking insects on Tabebuia ochracea. Biotropica 26:302–307
Rzedowski J (ed) (1978) Vegetación de México. Limusa, México
Ruiz-Guerra B, Guevara R, Mariano NA, Dirzo R (2010) Insect herbivory declines with forest fragmentation and covaries with plant regeneration mode: evidence from a Mexican tropical rain forest. Oikos 119:317–325
SAS (2000) Categorical data analysis using the SAS system. SAS Institute, Cary
Smith DM, Nufio CR (2004) Levels of herbivory in Two Costa Rican rain forests: implications for studies of fossil herbivory. Biotropica 36:318–326
SPSS, Science Marketing Department (1999) Sigma scan users guide. Science, Chicago
Strauss SY (1990) The role of plant genotype, environment and gender in resistance to a specialist chrysomelid herbivore. Oecologia 84:111–116
Strauss S, Agrawal A (1999) The ecology and evolution of tolerance to herbivory. Trends Ecol Evol 14:179–185
Torres AM, Mau-Lastovicka T, Rezaaiyan TR (1987) Total phenolics and high-performance liquid chromatography of phenolic acids of avocado. J Agric Food Chem 35:921–925
Uribe-Mú CA, Quesada M (2006) Preferences, patterns and consequences of branch removal on the dioecious tropical tree Spondias purpurea (Anacardiaceae) by the insect borer Oncideres albomarginata chamela (Cerambycidae). Oikos 112:691–697
Watson MA (1995) Sexual differences in plant developmental phenology affect plant-herbivore interactions. Trends Ecol Evol 10:180–182
Watterson JJ, Butler L (1983) Occurrence of an unusual leucoanthocyanidin and absence of proanthocyanidins in sorghum leaves. J Agric Food Chem 31:41–45
Wilson TC, Hagerman AE (1990) Quantitative determination of ellagic acid. J Agric Food Chem 38:678–1683
Zhuang XP, Lu YY, Yang GS (1992) Extraction and determination of flavonoids in ginkgo. Chin Herb Med 23:122–124
Acknowledgments
This work was supported by a Masters scholarship from the Consejo Nacional de Ciencia y Tecnología (CONACyT, No. 165050) grant to C.C.N. The authors thank Chamela Biological Station (UNAM) for collecting facilities. We are grateful to Posgrado en Ciencias Biológicas of Universidad Nacional Autónoma de México (UNAM).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Heikki M. T. Hokkanen.
Rights and permissions
About this article
Cite this article
Maldonado-López, Y., Cuevas-Reyes, P., Sánchez-Montoya, G. et al. Growth, plant quality and leaf damage patterns in a dioecious tree species: is gender important?. Arthropod-Plant Interactions 8, 241–251 (2014). https://doi.org/10.1007/s11829-014-9314-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-014-9314-3