Abstract
Amyotrophic lateral sclerosis (ALS) is the most frequent motor neuron disease, affecting the upper and/or lower motor neurons. However, extramotor symptoms can also occur; cognitive deficits are present in more than 40% of patients and 5–8% of ALS patients develop frontotemporal dementia. There is no effective treatment for ALS and median survival is 2–3 years after onset.
Amyotrophic lateral sclerosis is a genetically heterogeneous disorder with monogenic forms as well as complex genetic etiology. Currently, complex genetic risk factors are of minor interest for routine diagnostic testing or counseling of patients and their families. By contrast, a monogenic cause can be identified in 70% of familial and 10% of sporadic ALS cases. The most frequent genetic cause is a noncoding hexanucleotide repeat expansion in the C9orf72 gene. In recent years, high-throughput sequencing technologies have helped to identify additional monogenic and complex risk factors of ALS.
Genetic counseling should be offered to all ALS patients and their first- and possibly second-degree relatives, and should include information about the possibilities and limitations of genetic testing. Routine diagnostic testing should at least encompass the most frequently mutated disease genes (C9orf72, SOD1, TDP-43, FUS). Targeted sequencing approaches including further disease genes may be applied. Caution is warranted as the C9orf72 repeat expansion cannot be detected by routine sequencing technologies and testing by polymerase chain reaction (PCR) is failure-prone.
Predictive testing is possible in families in which a genetic cause has been identified, but the limitations of genetic testing (i. e., the problems of incomplete penetrance, variable expressivity and possible oligogenic inheritance) have to be explained to the families.
Zusammenfassung
Die amyotrophe Lateralsklerose (ALS) ist die häufigste neurodegenerative Erkrankung des motorischen Nervensystems. Ursächlich ist der Untergang des ersten und/oder zweiten Motoneurons, wobei weitere neuronale Strukturen betroffen sein können. Neben motorischen Befunden können z. B. kognitive Defizite bestehen (mehr als 40 % der Patienten); eine frontotemporale Demenz entwickelt sich bei 5–8 % aller Patienten. Eine ursächliche Therapie gibt es derzeit nicht, die mittlere Überlebenszeit beträgt 2–3 Jahre.
Die ALS ist eine genetisch heterogene Erkrankung mit monogenen Formen und komplex-genetischen Risikofaktoren. Letztere spielen für diagnostische Testungen und bei Beratungen von ALS-Patienten und ihren Familien z. Zt. (noch) eine untergeordnete Rolle. Bei 70 % der familiären und 10 % der sporadischen ALS-Patienten können genetische Untersuchungen dagegen die Diagnose einer monogenen ALS sichern. Eine nichtkodierende Hexanukleotidrepeat-Expansion des C9orf72-Gens ist dabei die häufigste genetische Ursache einer ALS. Hochdurchsatzsequenzierungen haben in den letzten Jahren wesentlich dazu beigetragen, weitere genetische Risikofaktoren der ALS zu identifizieren.
ALS-Patienten sowie erst- und ggf. zweitgradig Verwandten sollte eine genetische Beratung angeboten werden, in der die Möglichkeiten und Limitationen von genetischen Testungen erläutert werden. Eine routinediagnostische Testung sollte zumindest die häufigsten Krankheitsgene (C9orf72, SOD1, TDP-43, FUS) umfassen. Panelanalysen mit weiteren Genen können durchgeführt werden. Einschränkend ist zu erwähnen, dass die Repeatexpansion des C9orf72-Gens durch Sequenzierung in der Regel nicht nachweisbar ist und auch PCR-basierte Analyseverfahren fehleranfällig sind.
Eine gezielte prädiktive Testung ist im Falle eines Mutationsnachweises möglich, wobei die Grenzen der Aussagekraft des genetischen Tests (inkomplette und altersabhängige Penetranz, variable Expressivität, eventuelles Vorliegen mehrerer pathogener Mutationen bei einer Person) verdeutlicht werden müssen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction and clinical aspects
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that primarily affects the upper and lower motor neurons. The degeneration of the lower motor neurons results in the denervation of muscles followed by fasciculation, cramps, muscle wasting, and weakness. The degeneration of the upper motor neurons results in a loss of fine motor control of the lower motor neuron system, causing spastic paresis. The initial presentation varies between patients, depending on the affected motor neurons. Muscle paresis of the limbs is seen in spinal-onset disease (>75% of patients), whereas dysarthria is usually the first symptom in patients with bulbar onset.
Amyotrophic lateral sclerosis typically manifests later in life, with a peak incidence in the 7th decade, with males more frequently affected than females (1.5/1; [33]). Death typically occurs within 2–3 years of the onset of first symptoms, mainly due to respiratory failure, but overall survival time ranges from a few months to decades. In Europe, the incidence is about 2–3 per 100,000 individuals [19].
Although defined as a pure motor neuron disease by Charcot in 1869, ALS is now recognized as a multi-systemic disorder that may also affect frontotemporal, oculomotor, cerebellar, and/or sensory systems, and more rarely the basal ganglia and autonomic nervous system [35]. Around 10% of ALS patients fulfill the Neary criteria for frontotemporal dementia (FTD), whereas cognitive impairment with mainly executive dysfunction can be recognized in more than 40% of ALS patients [30]. Initially regarded as distinct diseases, both primary lateral sclerosis (PLS), which affects only upper motor neurons, and progressive muscular atrophy (PMA), which affects only lower motor neurons, are nowadays considered variants of ALS [27].
According to the revised Escorial criteria, diagnosis relies on the identification of upper and lower motor neuron signs in clinical, electrophysiological, and neuropathological examinations, as well as the progressive spread of signs, whereas differential diagnoses are excluded [27]. Treatable differential diagnoses include spinal stenosis, multifocal motor neuropathy, and myasthenia gravis. To date, there is no definitive diagnostic test for ALS, and the clinical diagnosis instead relies on clinical findings, electrophysiological results, and the exclusion of phenocopies. Although not integrated into standard clinical practice, several biomarkers such as cerebrospinal fluid (CSF) neurofilament levels are useful in supporting the diagnosis, particularly in patients with clinically doubtful signs of upper motor neuron involvement [46].
Amyotrophic lateral sclerosis patients should be managed by a multidisciplinary team, including neurologists, psychologists, physiotherapists, pulmonologists, speech specialists, and nutritionists [6]. Symptomatic treatment options include pharmacological and nonpharmacological approaches. For instance, spasticity can be addressed by administration of baclofen whereas hypersalivation can be treated with anticholinergic drugs or Botulinum toxin injection into the parotid glands. Pain, as commonly reported by ALS patients, is treated according to the WHO’s pain relief ladder. Dietary changes (e. g., fluid thickeners) can help to improve nutrition and a gastrostomy tube is an option if severe dysphagia is present. Speech therapy is frequently necessary and assisted communication (customized software) can also be used. Non-invasive ventilation is the preferred treatment for respiratory insufficiency. As substantial immobility and loss of speech are the major problems in advanced disease stages, the patients’ individual wishes for life-prolonging therapies (such as tracheostomy) should be addressed at early disease stages in end-of-life discussions, as cognitive or communication difficulties may arise over time.
In most ALS patients the disease cause is unknown. In up to 25% of cases, however, patients have a family history, with close relatives affected by ALS or FTD. Genetic causes have been identified in sporadic as well as familial cases. This review gives an overview of the most frequently as well as newly identified monogenic causes as well as genetic risk factors of ALS and will discuss ALS-specific aspects of genetic counseling of patients and their families.
Monogenic causes of ALS
Unraveling the genetic basis of ALS has provided fundamental insights into the pathophysiology of not only familial ALS (FALS), but also of sporadic ALS (SALS). Pathways involved include aberrant RNA metabolism and nucleocytoplasmic transport as well as impaired protein homeostasis. It took until 2011 to identify the most commonly mutated ALS gene, i. e., C9orf72. SOD1, the second most common and ALS gene to be identified was already linked to ALS in 1993. Both genes together account for around 40% of FALS cases. With the advent of next-generation sequencing techniques, additional disease genes have been found and at present, a genetic cause is found in about 70% of FALS patients and 10% of SALS patients [31]. However, clinically valid frequency data, especially on the more recently identified disease genes, are scarce. The genes most frequently implicated in ALS are inherited in an autosomal-dominant manner with age-dependent penetrance. An overview of all currently known monogenic causes is given in Table 1.
Chromosome 9 open reading frame 72
A hexanucleotide (GGGGCC-) repeat expansion in the noncoding region of the gene chromosome 9 open reading frame 72 (C9orf72) is the most frequent cause of FALS and FTD. A common genetic cause of ALS and FTD was indeed first proposed in 1991 by linkage analyses, and later genome-wide association studies (GWAS) also pointed to a common underlying genetic factor located in chromosomal region 9p21.2 [24, 39]. Although the exact cut-off between normal alleles and pathogenic expanded alleles is still unclear, repeat expansions with several hundred or thousand repeats are thought to be pathogenic. In European populations, a C9orf72 repeat expansion was shown to be the underlying genetic cause in up to 35% of FALS and could also be detected in around 5% of SALS patients [50]. C9orf72-related diseases include primarily pure ALS, pure FTD or a mixed phenotype of both. C9orf72 repeat expansions have also rarely been identified in other neurodegenerative diseases, such as Huntington disease-like disorders [20]. Bulbar onset has been more frequently observed in C9orf72-related ALS [21].
It is still unclear whether anticipation—as observed in other repeat diseases—also exists in C9orf72-associated diseases. Somatic and intergenerational repeat instabilities [21] have been observed: one study reported a repeat expansion of around 70 repeats in a healthy father and an increase to around 1750 repeats in his affected children [45]. Conversely, we observed an expanded allele of 1800–2400 repeats in an affected father and a so-called intermediate allele of 100–120 repeats in the healthy son (unpublished data).
Disease penetrance of C9orf72-related ALS is thought to be nearly 100% by the age of 80. No prediction of the individual phenotype, i. e., ALS, FTD or ALS/FTD, the exact age at onset, the disease course, and disease duration is currently possible.
To detect the C9orf72 repeat expansion, PCR-based amplicon fragment analyses, repeat-primed PCR protocols, and Southern blotting are used. The gold standard for detecting the C9orf72 repeat expansion is Southern blotting, as PCR-based techniques are still failure-prone [1].
The molecular mechanisms underlying neurodegeneration in C9orf72-related diseases are a matter of debate and several different, nonmutually exclusive pathomechanisms have been described. The C9orf72 repeat expansion may be associated with aberrant RNA metabolism because of sequestration of RNA binding proteins, production of abnormal RNA species, or increasing DNA instability due to the formation of RNA–DNA hybrid structures [42]. Altered protein homeostasis may result from impaired autophagy or accumulation of dipeptide repeat proteins after non-ATG mediated translation [25]. Dipeptide repeat proteins have also been shown to interfere with nucleocytoplasmic transport [48]. Last but not least, haploinsufficiency could be another explanation [34].
Superoxide dismutase 1
The superoxide dismutase 1 (SOD1) protein is ubiquitously expressed. It encodes a homodimeric enzyme that catalyzes the reduction of superoxide anions (a reactive oxygen species) to oxygen and hydrogen peroxide. Most SOD1 mutations reported to date are missense mutations, but smaller deletions and duplications have also been detected [4]. Mutations in SOD1 are found in around 15% of FALS patients and up to 2% of SALS patients [50]. Most mutations in SOD1 have been identified in families with autosomal-dominant ALS. However, the common mutation D90A (according to HGVS c.272A>C p.Asp91Ala, NM_000454) can also be inherited in an autosomal-recessive manner [5]. Recently, emerging evidence suggests that neuronal aggregates of misfolded SOD1 protein might have prion-like properties and cause a fulminant ALS-like phenotype when injected intraspinally in minute amounts into 100-day-old healthy mice [7].
TAR DNA binding protein and FUSED IN SARCOMA
Accumulation of proteinaceous inclusions in motor neurons are a neuropathological hallmark of ALS and TDP43-postive inclusions can be detected in around 95% of all ALS patients [26]. However, TDP43 mutations can be identified in only a few patients. TDP43 mutations account for around 4% of FALS and 1% of SALS cases [50]. Most mutations published to date (Human Gene Mutation Database, HGMD, n = 44) are missense mutations and are mainly located within the C‑terminal part of the protein. TAR DNA-binding protein 43 (TDP43) as well as FUSED IN SARCOMA (FUS) are RNA-binding proteins and have been shown to mislocalize from the nuclear to the cytoplasmic compartment when mutated. A loss of normal processing of their target RNA is one of the hypothesized pathomechanisms [3, 49]. Both proteins also contain prion-like domains, which may also represent a disease mechanism. Mutations in FUS can be identified in around 5% of FALS and 0.5% of SALS patients [50]. Of note, FUS mutation frequency is especially high in sporadic, early onset (<35 years of age) ALS patients because of de novo mutations [22]. On the contrary, there are no convincing data concerning a major contribution of de novo mutations in additional genes in ALS pathogenesis [16], although this has been claimed by initial studies [12, 37].
Novel disease genes
Within the last 5 years, nine novel genes associated with monogenic forms of ALS have been identified: KIF5A, CCNF, NEK1, TBK1, MATR3, TUBA4A, CHCHD10, HNRNPA1, and HNRNPA2B1 [10, 13, 43]. Frequency data on mutations in these genes are scarce and rough estimates exist for only eight of these genes (KIF5A, CCNF, NEK1, TBK1, MATR3, TUBA4A, CHCHD10).
Missense mutations in KIF5A’s N‑terminal motor domain or coiled-coil domain and heterozygous de novo frame-shift mutations in its C‑terminal part were associated with hereditary spastic paraplegia 10, Charcot–Marie–Tooth disease type 2 (CMT2), and neonatal intractable myoclonus respectively. Very recently, KIF5A was also implicated in ALS [10]. Two splice-site mutations and a rare missense mutation, leading to loss of mutant RNA or aberrant splicing, were identified in FALS patients, segregating with the phenotype within the families. Furthermore, a single non-synonymous SNP (rs113247976, c.2957C>T p.Pro986Leu) was significantly enriched in FALS patients (3.40% vs 1% in controls P = 1.28 × 10−7; [10]).
Mutations in CCNF were identified in both ALS and FTD, and are thought to account for 4% of FALS and 2% of SALS cases [43]. NEK1 mutations were associated with ALS without dementia and found in up to 2% of ALS cases [9]. By burden analysis, nonsynonymous variants in TBK1 were found to be enriched in ALS patients, but the number of TBK1-related ALS is low (around 1% of FALS and 1% of SALS cases; [15, 18]). Even less frequently, mutations in MATR3, TUBA4A, and CHCHD10 can be detected [13].
The proteins encoded by these recently identified disease genes are involved in several intracellular pathways known to be implicated in ALS or interact with known ALS genes. MATR3 was shown to interact with FUS and TDP43 and regulate gene expression [23, 47]. CHCHD10 is localized to the mitochondrial intermembraneous space and is important for mitochondrial maintenance. Some data suggest that CHCHD10 pathology is caused by haploinsufficiency [11]. CHCHD10 also interacts with TDP43 and promotes its retention to the nucleus [44]. Missense mutations in TUBA4A prevent the encoded alpha tubulin from polymerizing and result in a disturbed microtubule network [36]. TBK1 interacts with several proteins and is involved in cellular processes such as autophagy, neuroinflammation, and ubiquitin-mediated protein degradation [18]. Also, CCNF was shown to be part of the ubiquitin-dependent protein degradation processes [43].
Genetic risk factors
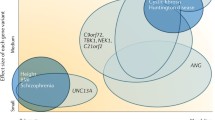
Amyotrophic lateral sclerosis is a genetically heterogeneous disorder with monogenic as well as multifactorial inheritance, and boundaries are fluid. Some genetic risk factors for ALS have been identified by genome-wide association studies (GWAS). It is suggested that around 8.5% of heritability of ALS is tagged by common SNPs. However, only 0.2% of variance in liability can currently be explained by six common susceptibility loci in/near the genes UNC13A, SARM1, MOBP, SCFD1, C21orf2, and C9orf72 [32]. For the latter, several SNPs have been shown to contribute to a disease haplotype that influences the length of the hexanucleotide repeat [29]. In the future, increasing sample sizes may help to unravel more SNP-based heritability in ALS.
TDP43 toxicity was shown to be increased in yeast by expression of Ataxin 2 (ATXN2). Indeed, in humans, intermediate-length polyglutamine stretches (27–33 glutamines) confer a risk for ALS, whereas repeat stretches larger than 34 glutamine repeat are associated with spinocerebellar ataxia type 2 [17]. With the exception of research projects, genetic testing of (complex) genetic risk factors is of minor clinical relevance to date.
Genetic testing and counseling
The diagnosis of ALS is based on clinical and neurophysiological findings. With new sequencing technologies, clinical genetic testing becomes more feasible and offers the possibility of a definitive diagnosis in a growing number of cases [40]. The probability of identifying a genetic cause is higher in familial than in sporadic ALS. Indeed, a genetic cause in seemingly sporadic ALS can be masked by recessive inheritance, reduced penetrance, small family size, lack of family information, and illegitimacy. Within the last few years, neurologists started offering genetic counseling to ALS patients more frequently and patients who underwent genetic counseling reported a positive experience and found value in testing [41]. According to the guidelines of the German Society of Neurology, genetic testing should be offered to patients with familial ALS, patients who have family members with dementia, and patients with an early onset and rapid progression [28]. More recent recommendations suggested diagnostic C9orf72 testing in all ALS patients, regardless of family history [14].
In a first diagnostic step, i. e., before multigene sequencing approaches are applied, a repeat expansion in C9orf72 as the most frequent genetic cause of ALS should be excluded. The genes tested in custom-made panels vary depending on the laboratory. In a routine clinical setting, we suggest that at least the most frequent disease genes, such as SOD1, FUS, and TPD43, should be tested in all patients. For research purposes and maybe also in clinical settings, further genes may be analyzed. But especially for recently identified genes, variant interpretation may be challenging. Obviously, the identification of variants of unknown significance is more probable in newer disease genes.
In around 5% of families with FALS, a pathogenic mutation in more than one ALS gene could be detected, providing evidence for oligogenic inheritance in ALS [8].
Owing to the absence of preventive medical treatment and the natural disease course of ALS, predictive genetic testing is challenging and shares similarities with other neurodegenerative disorders, such as Huntington disease. Variable expressivity, age-dependent penetrance, and oligogenic inheritance further complicate genetic counseling in healthy relatives who seek to know about their disease risks. In a survey of neurologists specialized in ALS, only 52% stated they offer predictive testing to ALS relatives and one-fifth of neurologists who would offer predictive testing would not recommend it to their own family [38]. However, formal provision of genetic testing for those who have a first- or second-degree relative with ALS is recommended [14]. Given the aforementioned complexities, one might argue against predictive testing in ALS. But for the individual case, there may be several personal reasons why one might opt for predictive testing, such as life decisions like going on a round-the-world trip with a caravan before or after retirement (as reported by a 57-year old woman whose sisters both carried a SOD1 mutation and developed ALS by the age of 63 and 61 years respectively). Younger individuals may choose not to have children or ask for preimplantation genetic testing. Most people express that the anxiety of living without knowing is worse than living with the knowledge. In our experience, a clear communication of the genetic complexity in ALS is of utmost importance for people undergoing predictive testing. It must be clearly mentioned that genetic testing only addresses the genetic alteration already documented in the family and does not test all known genetic variants in ALS. Issues linked to variable expressivity and age-dependent penetrance should be communicated. Similar to predictive testing in HD, multiple counseling sessions including predecision, pre-test, and post-test counseling sessions are desirable. Additional counseling sessions should be offered if necessary. Obviously, the decision to undergo testing should be voluntary, and informed consent as well as psychological readiness (exclusion of active psychiatric conditions) are prerequisites. Consulters may decide at any time not to receive or to delay receiving the test results. Detailed recommendations for predictive testing of ALS were recently published [14].
Outlook
The knowledge of the biological and genetic basis of ALS and the advances in the care of ALS patients have improved substantially within the last few years. Genetic causes of ALS have been identified in both sporadic and familial patients and the number of disease genes involved is still increasing. One might guess that most of the monogenic forms are already identified, but there is still “missing heritability,” which might be explained by rare variants with large effect sizes. The identification of genetic causes of ALS will help to develop new therapeutic approaches, either by the identification of shared disease pathways such as TDP43 pathology or by targeted therapies for known mutations. Currently, antisense oligonucleotide trials in SOD1- and C9orf72-related ALS are being conducted. Besides riluzole, the second modifying medication, edaravone, became FDA-approved last year. Whether it is beneficial to all ALS patients or just to subgroups needs to be evaluated within the next few years [2]. In the future, biomarkers will hopefully help to monitor disease progression and genomics or transcriptomics will help to further personalize treatment to one’s individual disease subtype.
References
Akimoto C et al (2014) A blinded international study on the reliability of genetic testing for GGGGCC-repeat expansions in C9orf72 reveals marked differences in results among 14 laboratories. J Med Genet 51(6):419–424
Al-Chalabi A et al (2017) July 2017 ENCALS statement on edaravone. Amyotroph Lateral Scler Frontotemporal Degener 18(7–8):471–474
Amlie-Wolf A et al (2015) Transcriptomic changes due to cytoplasmic TDP-43 expression reveal dysregulation of Histone transcripts and nuclear chromatin. PLoS ONE 10(10):e141836
Andersen PM, Al-Chalabi A (2011) Clinical genetics of amyotrophic lateral sclerosis: what do we really know? Nat Rev Neurol 7(11):603–615
Andersen PM et al (1996) Autosomal recessive adult-onset amyotrophic lateral sclerosis associated with homozygosity for Asp90Ala CuZn-superoxide dismutase mutation. A clinical and genealogical study of 36 patients. Brain 119(Pt 4):1153–1172
Van den Berg JP et al (2005) Multidisciplinary ALS care improves quality of life in patients with ALS. Neurology 65(8):1264–1267
Bidhendi EE et al (2016) Two superoxide dismutase prion strains transmit amyotrophic lateral sclerosis-like disease. J Clin Invest 126(6):2249–2253
van Blitterswijk M et al (2012) Evidence for an oligogenic basis of amyotrophic lateral sclerosis. Hum Mol Genet 21(17):3776–3784
Brenner D et al (2016) NEK1 mutations in familial amyotrophic lateral sclerosis. Brain 139(Pt 5):e28
Brenner D et al (2018) Hot-spot KIF5A mutations cause familial ALS. Brain. https://doi.org/10.1093/brain/awx370
Brockmann SJ et al (2018) CHCHD10 mutations p.R15L and p.G66V cause motoneuron disease by haploinsufficiency. Hum Mol Genet 27(4):706–715
Chesi A et al (2013) Exome sequencing to identify de novo mutations in sporadic ALS trios. Nat Neurosci 16(7):851–855
Chia R, Chio A, Traynor BJ (2018) Novel genes associated with amyotrophic lateral sclerosis: diagnostic and clinical implications. Lancet Neurol 17(1):94–102
Chio A et al (2014) Genetic counselling in ALS: facts, uncertainties and clinical suggestions. J Neurol Neurosurg Psychiatry 85(5):478–485
Cirulli ET et al (2015) Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science 347(6229):1436–1441
van Doormaal PTC et al (2017) The role of de novo mutations in the development of amyotrophic lateral sclerosis. Hum Mutat 38(11):1534–1541
Elden AC et al (2010) Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature 466(7310):1069–1075
Freischmidt A et al (2015) Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nat Neurosci 18(5):631–636
Hardiman O et al (2017) The changing picture of amyotrophic lateral sclerosis: lessons from European registers. J Neurol Neurosurg Psychiatr 88(7):557–563
Hensman Moss DJ et al (2014) C9orf72 expansions are the most common genetic cause of Huntington disease phenocopies. Neurology 82(4):292–299
Hubers A et al (2014) Polymerase chain reaction and Southern blot-based analysis of the C9orf72 hexanucleotide repeat in different motor neuron diseases. Neurobiol Aging 35(5):1214e1–1214e6
Hubers A et al (2015) De novo FUS mutations are the most frequent genetic cause in early-onset German ALS patients. Neurobiol Aging 36(11):3117.e1–3117.e6
Johnson JO et al (2014) Mutations in the Matrin 3 gene cause familial amyotrophic lateral sclerosis. Nat Neurosci 17(5):664–666
Laaksovirta H et al (2010) Chromosome 9p21 in amyotrophic lateral sclerosis in Finland: a genome-wide association study. Lancet Neurol 9(10):978–985
Lehmer C et al (2017) Poly-GP in cerebrospinal fluid links C9orf72-associated dipeptide repeat expression to the asymptomatic phase of ALS/FTD. EMBO Mol Med 9(7):859–868
Ludolph AC, Brettschneider J (2015) TDP-43 in amyotrophic lateral sclerosis—is it a prion disease? Eur J Neurol 22(5):753–761
Ludolph A et al (2015) A revision of the El Escorial criteria—2015. Amyotroph Lateral Scler Frontotemporal Degener 16(5–6):291–292
Ludolph AC et al (2015) S1-Leitlinie Amyotrophe Lateralsklerose (Motoneuronerkrankungen). In: Deutsche Gesellschaft für Neurologie (Hrsg) Leitlinien für Diagnostik und Therapie in der Neurologie. www.dgn.org/leitlinien. Zugegriffen: 09.07.2018
Ng ASL, Tan EK (2017) Intermediate C9orf72 alleles in neurological disorders: does size really matter? J Med Genet 54(9):591–597
Phukan J et al (2012) The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population-based study. J Neurol Neurosurg Psychiatr 83(1):102–108
Renton AE, Chio A, Traynor BJ (2014) State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci 17(1):17–23
van Rheenen W et al (2016) Genome-wide association analyses identify new risk variants and the genetic architecture of amyotrophic lateral sclerosis. Nat Genet 48(9):1043–1048
Rosenbohm A et al (2018) Phenotypic differences of amyotrophic lateral sclerosis (ALS) in China and Germany. J Neurol 265(4):774. https://doi.org/10.1007/s00415-018-8735-9
Shi Y et al (2018) Haploinsufficiency leads to neurodegeneration in C9ORF72 ALS/FTD human induced motor neurons. Nat Med. https://doi.org/10.1038/nm.4490
Silani V, Ludolph A, Fornai F (2017) The emerging picture of ALS: a multisystem, not only a “motor neuron disease”. Arch Ital Biol 155(4):99–109
Smith BN et al (2014) Exome-wide rare variant analysis identifies TUBA4A mutations associated with familial ALS. Neuron 84(2):324–331
Steinberg KM et al (2015) Exome sequencing of case-unaffected-parents trios reveals recessive and de novo genetic variants in sporadic ALS. Sci Rep 5:9124
Vajda A et al (2017) Genetic testing in ALS: a survey of current practices. Neurology 88(10):991–999
Vance C et al (2006) Familial amyotrophic lateral sclerosis with frontotemporal dementia is linked to a locus on chromosome 9p13.2-21.3. Brain 129(Pt 4):868–876
Volk AE, Kubisch C (2017) The rapid evolution of molecular genetic diagnostics in neuromuscular diseases. Curr Opin Neurol 30(5):523–528
Wagner KN et al (2018) Patients with sporadic and familial amyotrophic lateral sclerosis found value in genetic testing. Mol Genet Genomic Med 6(2):224. https://doi.org/10.1002/mgg3.360
Walker C et al (2017) C9orf72 expansion disrupts ATM-mediated chromosomal break repair. Nat Neurosci 20(9):1225–1235
Williams KL et al (2016) CCNF mutations in amyotrophic lateral sclerosis and frontotemporal dementia. Nat Commun 7:11253
Woo JA et al (2017) Loss of function CHCHD10 mutations in cytoplasmic TDP-43 accumulation and synaptic integrity. Nat Commun 8:15558
Xi Z et al (2015) Jump from pre-mutation to pathologic expansion in C9orf72. Am J Hum Genet 96(6):962–970
Xu Z et al (2016) Neurofilaments as Biomarkers for Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. PLoS ONE 11(10):e164625
Yamaguchi A, Takanashi K (2016) FUS interacts with nuclear matrix-associated protein SAFB1 as well as Matrin3 to regulate splicing and ligand-mediated transcription. Sci Rep 6:35195
Zhang K et al (2015) The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature 525(7567):56–61
Zhou Y et al (2014) FUS-regulated RNA metabolism and DNA damage repair: Implications for amyotrophic lateral sclerosis and frontotemporal dementia pathogenesis. Rare Dis 2:e29515. https://doi.org/10.4161/rdis.29515
Zou ZY et al (2017) Genetic epidemiology of amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatr 88(7):540–549
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A.E. Volk, J.H. Weishaupt, P.M. Andersen, A.C. Ludolph and C. Kubisch declare that they have no competing interests.
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Volk, A.E., Weishaupt, J.H., Andersen, P.M. et al. Current knowledge and recent insights into the genetic basis of amyotrophic lateral sclerosis. medgen 30, 252–258 (2018). https://doi.org/10.1007/s11825-018-0185-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11825-018-0185-3